Association of Oxidative Stress with Kidney Injury in a Hyperandrogenemic Female Rat Model
- PMID: 36895462
- PMCID: PMC9989239
- DOI: 10.30476/IJMS.2022.93594.2497
Association of Oxidative Stress with Kidney Injury in a Hyperandrogenemic Female Rat Model
Abstract
Background: Polycystic ovary syndrome (PCOS) is the most common reproductive dysfunction in premenopausal women. PCOS is associated with oxidative stress (OS), which is the main risk factor for renal diseases. This study aimed to investigate the mechanisms responsible for renal injury in a hyperandrogenemic female rat model.
Methods: This study was conducted from December 2019 to September 2021 at Shiraz Nephro-Urology Research Centre, Shiraz University of Medical Sciences (Shiraz, Iran). Thirty female Sprague-Dawley rats were randomly divided into three groups (n=10), namely control, sham, and dehydroepiandrosterone (DHEA). Plasma total testosterone, plasma creatinine (Cr), and blood urea nitrogen (BUN) levels were measured. In addition, total oxidant status (TOS), total antioxidant capacity (TAC), oxidative stress index (OSI), and histopathological changes in the ovaries and kidneys were determined. Data were analyzed using the GraphPad Prism software, and P<0.05 was considered statistically significant.
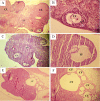
Results: Plasma total testosterone levels increased by nine-fold in DHEA-treated rats compared to controls (P=0.0001). Administration of DHEA increased Cr and BUN levels and caused severe renal tubular cell injury. In addition, plasma and tissue (kidney and ovary) TAC levels decreased significantly, but TOS levels and OSI values were significantly increased (P=0.019). Significant damage to both glomerular and tubular parts of the kidney and ovarian follicular structure was observed in the DHEA group.
Conclusion: Hyperandrogenemia caused systemic abnormalities through OS-related mechanisms and damaged renal and ovarian tissues. DHEA treatment in rat models is recommended to study the mechanisms that mediate PCOS-associated renal injury.
Keywords: Dehydroepiandrosterone; Kidney disease; Oxidative stress; Polycystic ovary syndrome.
Copyright: © Iranian Journal of Medical Sciences.
Conflict of interest statement
None declared.
Figures


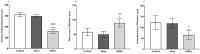

Similar articles
-
The use of dehydroepiandrosterone-treated rats is not a good animal model for the study of metabolic abnormalities in polycystic ovary syndrome.Taiwan J Obstet Gynecol. 2018 Oct;57(5):696-704. doi: 10.1016/j.tjog.2018.08.015. Taiwan J Obstet Gynecol. 2018. PMID: 30342654
-
[Study on the oxidative stress in the ovaries of a rat model of polycystic ovary].Sichuan Da Xue Xue Bao Yi Xue Ban. 2015 Mar;46(2):238-42, 247. Sichuan Da Xue Xue Bao Yi Xue Ban. 2015. PMID: 25924437 Chinese.
-
Ovarian Metabolic activity in Dehydroepiandrosterone-Induced Polycystic Ovary in Wistar rats Treated with Aspirin.JBRA Assist Reprod. 2020 Jan 30;24(1):41-54. doi: 10.5935/1518-0557.20190059. JBRA Assist Reprod. 2020. PMID: 31608617 Free PMC article.
-
Polycystic ovarian disease: the adrenal connection.Pediatr Endocrinol Rev. 2006 Jan;3 Suppl 1:205-7. Pediatr Endocrinol Rev. 2006. PMID: 16641861 Review.
-
OXIDATIVE STRESS AND REPRODUCTIVE FUNCTION: Oxidative stress in polycystic ovary syndrome.Reproduction. 2022 Nov 22;164(6):F145-F154. doi: 10.1530/REP-22-0152. Print 2022 Dec 1. Reproduction. 2022. PMID: 36279177 Review.
Cited by
-
Association between the oxidative balance score and estimated glomerular filtration rate: 2007-2018 NHANES.Heliyon. 2024 Oct 11;10(20):e39230. doi: 10.1016/j.heliyon.2024.e39230. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39640760 Free PMC article.
-
Trinitroglycerine-loaded chitosan nanoparticles attenuate renal ischemia-reperfusion injury by modulating oxidative stress.Sci Rep. 2024 Dec 30;14(1):32112. doi: 10.1038/s41598-024-83886-3. Sci Rep. 2024. PMID: 39738455 Free PMC article.
-
Renometabolic disorder in experimental rat model of polycystic ovarian syndrome is reversed by acetate-mediated inhibition of pyruvate dehydrogenase kinase 4.BMC Nephrol. 2025 May 13;26(1):234. doi: 10.1186/s12882-025-04157-5. BMC Nephrol. 2025. PMID: 40361039 Free PMC article.
-
Long-term effects of warm water immersion on kidney tissue damage in diabetic rats.Iran J Basic Med Sci. 2024;27(9):1162-1171. doi: 10.22038/IJBMS.2024.74307.16141. Iran J Basic Med Sci. 2024. PMID: 39055872 Free PMC article.
-
Oxidative stress and energy metabolism abnormalities in polycystic ovary syndrome: from mechanisms to therapeutic strategies.Reprod Biol Endocrinol. 2024 Dec 26;22(1):159. doi: 10.1186/s12958-024-01337-0. Reprod Biol Endocrinol. 2024. PMID: 39722030 Free PMC article. Review.
References
-
- Chappell NR, Gibbons WE, Blesson CS. Pathology of hyperandrogenemia in the oocyte of polycystic ovary syndrome. Steroids. 2022;180:108989. doi: 10.1016/j.steroids.2022.108989. [ PMC Free Article ] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
