Haploidentical hematopoietic stem cell transplantation as individual treatment option in pediatric patients with very high-risk sarcomas
- PMID: 36895486
- PMCID: PMC9990259
- DOI: 10.3389/fonc.2023.1064190
Haploidentical hematopoietic stem cell transplantation as individual treatment option in pediatric patients with very high-risk sarcomas
Abstract
Background: Prognosis of children with primary disseminated or metastatic relapsed sarcomas remains dismal despite intensification of conventional therapies including high-dose chemotherapy. Since haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is effective in the treatment of hematological malignancies by mediating a graft versus leukemia effect, we evaluated this approach in pediatric sarcomas as well.
Methods: Patients with bone Ewing sarcoma or soft tissue sarcoma who received haplo-HSCT as part of clinical trials using CD3+ or TCRα/β+ and CD19+ depletion respectively were evaluated regarding feasibility of treatment and survival.
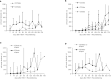
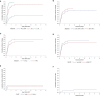
Results: We identified 15 patients with primary disseminated disease and 14 with metastatic relapse who were transplanted from a haploidentical donor to improve prognosis. Three-year event-free survival (EFS) was 18,1% and predominantly determined by disease relapse. Survival depended on response to pre-transplant therapy (3y-EFS of patients in complete or very good partial response: 36,4%). However, no patient with metastatic relapse could be rescued.
Conclusion: Haplo-HSCT for consolidation after conventional therapy seems to be of interest for some, but not for the majority of patients with high-risk pediatric sarcomas. Evaluation of its future use as basis for subsequent humoral or cellular immunotherapies is necessary.
Keywords: Ewing sarcoma; haploidentical hematopoietic stem cell transplantation; pediatric sarcoma; rhabdomyosarcoma; soft tissue sarcoma.
Copyright © 2023 Eichholz, Döring, Giardino, Gruhn, Seitz, Flaadt, Schwinger, Ebinger, Holzer, Mezger, Teltschik, Sparber-Sauer, Koscielniak, Abele, Handgretinger and Lang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Erdmann F, Kaatsch P, Grabow D, Spix C, German Childhood Cancer Registry - Annual Report 2019 (1980-2018) . Institute of medical biostatistics, epidemiology and informatics (IMBEI) at the university medical center of the Johannes Gutenberg university mainz. (2020). Deutsches Kinderkrebsregister, Mainz:
-
- Breneman JC, Lyden E, Pappo AS, Link MP, Anderson JR, Parham DM, et al. . Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma–a report from the intergroup rhabdomyosarcoma study IV. J Clin Oncol (2003) 21(1):78–84. doi: 10.1200/JCO.2003.06.129 - DOI - PubMed
-
- Koscielniak E, Klingebiel TH, Peters C, Hermann J, Burdach ST, Bender-Gotze C, et al. . Do patients with metastatic and recurrent rhabdomyosarcoma benefit from high-dose therapy with hematopoietic rescue? Report of the German/Austrian pediatric bone marrow transplantation group. Bone Marrow Transplant (1997) 19(3):227–31. doi: 10.1038/sj.bmt.1700628 - DOI - PubMed
-
- Koscielniak E, Gross-Wieltsch U, Treuner J, Winkler P, Klingebiel T, Lang P, et al. . Graft-versus-Ewing sarcoma effect and long-term remission induced by haploidentical stem-cell transplantation in a patient with relapse of metastatic disease. J Clin Oncol (2005) 23(1):242–4. doi: 10.1200/JCO.2005.05.940 - DOI - PubMed
LinkOut - more resources
Full Text Sources

