Haploidentical hematopoietic stem cell transplantation as individual treatment option in pediatric patients with very high-risk sarcomas
- PMID: 36895486
- PMCID: PMC9990259
- DOI: 10.3389/fonc.2023.1064190
Haploidentical hematopoietic stem cell transplantation as individual treatment option in pediatric patients with very high-risk sarcomas
Abstract
Background: Prognosis of children with primary disseminated or metastatic relapsed sarcomas remains dismal despite intensification of conventional therapies including high-dose chemotherapy. Since haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is effective in the treatment of hematological malignancies by mediating a graft versus leukemia effect, we evaluated this approach in pediatric sarcomas as well.
Methods: Patients with bone Ewing sarcoma or soft tissue sarcoma who received haplo-HSCT as part of clinical trials using CD3+ or TCRα/β+ and CD19+ depletion respectively were evaluated regarding feasibility of treatment and survival.
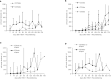
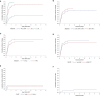
Results: We identified 15 patients with primary disseminated disease and 14 with metastatic relapse who were transplanted from a haploidentical donor to improve prognosis. Three-year event-free survival (EFS) was 18,1% and predominantly determined by disease relapse. Survival depended on response to pre-transplant therapy (3y-EFS of patients in complete or very good partial response: 36,4%). However, no patient with metastatic relapse could be rescued.
Conclusion: Haplo-HSCT for consolidation after conventional therapy seems to be of interest for some, but not for the majority of patients with high-risk pediatric sarcomas. Evaluation of its future use as basis for subsequent humoral or cellular immunotherapies is necessary.
Keywords: Ewing sarcoma; haploidentical hematopoietic stem cell transplantation; pediatric sarcoma; rhabdomyosarcoma; soft tissue sarcoma.
Copyright © 2023 Eichholz, Döring, Giardino, Gruhn, Seitz, Flaadt, Schwinger, Ebinger, Holzer, Mezger, Teltschik, Sparber-Sauer, Koscielniak, Abele, Handgretinger and Lang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Erdmann F, Kaatsch P, Grabow D, Spix C, German Childhood Cancer Registry - Annual Report 2019 (1980-2018) . Institute of medical biostatistics, epidemiology and informatics (IMBEI) at the university medical center of the Johannes Gutenberg university mainz. (2020). Deutsches Kinderkrebsregister, Mainz:
-
- Breneman JC, Lyden E, Pappo AS, Link MP, Anderson JR, Parham DM, et al. Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma–a report from the intergroup rhabdomyosarcoma study IV. J Clin Oncol (2003) 21(1):78–84. doi: 10.1200/JCO.2003.06.129 - DOI - PubMed
-
- Koscielniak E, Klingebiel TH, Peters C, Hermann J, Burdach ST, Bender-Gotze C, et al. Do patients with metastatic and recurrent rhabdomyosarcoma benefit from high-dose therapy with hematopoietic rescue? Report of the German/Austrian pediatric bone marrow transplantation group. Bone Marrow Transplant (1997) 19(3):227–31. doi: 10.1038/sj.bmt.1700628 - DOI - PubMed
-
- Koscielniak E, Gross-Wieltsch U, Treuner J, Winkler P, Klingebiel T, Lang P, et al. Graft-versus-Ewing sarcoma effect and long-term remission induced by haploidentical stem-cell transplantation in a patient with relapse of metastatic disease. J Clin Oncol (2005) 23(1):242–4. doi: 10.1200/JCO.2005.05.940 - DOI - PubMed
LinkOut - more resources
Full Text Sources

