Characterization of substrates and inhibitors of the human heterodimeric transporter 4F2hc-LAT1 using purified protein and the scintillation proximity radioligand binding assay
- PMID: 36895635
- PMCID: PMC9989278
- DOI: 10.3389/fphys.2023.1148055
Characterization of substrates and inhibitors of the human heterodimeric transporter 4F2hc-LAT1 using purified protein and the scintillation proximity radioligand binding assay
Abstract
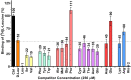
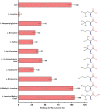
Amino acids have diverse and essential roles in many cellular functions such as in protein synthesis, metabolism and as precursors of different hormones. Translocation of amino acids and derivatives thereof across biological membranes is mediated by amino acid transporters. 4F2hc-LAT1 is a heterodimeric amino acid transporter that is composed of two subunits belonging to the SLC3 (4F2hc) and SLC7 (LAT1) solute carrier families. The ancillary protein 4F2hc is responsible for the correct trafficking and regulation of the transporter LAT1. Preclinical studies have identified 4F2hc-LAT1 as a valid anticancer target due to its importance in tumor progression. The scintillation proximity assay (SPA) is a valuable radioligand binding assay that allows the identification and characterization of ligands of membrane proteins. Here, we present a SPA ligand binding study using purified recombinant human 4F2hc-LAT1 protein and the radioligand [3H]L-leucine as tracer. Binding affinities of different 4F2hc-LAT1 substrates and inhibitors determined by SPA are comparable with previously reported K m and IC 50 values from 4F2hc-LAT1 cell-based uptake assays. In summary, the SPA is a valuable method for the identification and characterization of ligands of membrane transporters including inhibitors. In contrast to cell-based assays, where the potential interference with other proteins such as endogenous transporters persists, the SPA uses purified protein making target engagement and characterization of ligands highly reliable.
Keywords: JPH203; LAT1; Pichia pastoris; SLC7; amino acid transporter; inhibitor; membrane protein; scintillation proximity assay.
Copyright © 2023 Kantipudi, Harder and Fotiadis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Yeast Cell-Based Transport Assay for the Functional Characterization of Human 4F2hc-LAT1 and -LAT2, and LAT1 and LAT2 Substrates and Inhibitors.Front Mol Biosci. 2021 May 28;8:676854. doi: 10.3389/fmolb.2021.676854. eCollection 2021. Front Mol Biosci. 2021. PMID: 34124158 Free PMC article.
-
Pichia pastoris and the Recombinant Human Heterodimeric Amino Acid Transporter 4F2hc-LAT1: From Clone Selection to Pure Protein.Methods Protoc. 2021 Jul 24;4(3):51. doi: 10.3390/mps4030051. Methods Protoc. 2021. PMID: 34449687 Free PMC article.
-
The Heavy Chain 4F2hc Modulates the Substrate Affinity and Specificity of the Light Chains LAT1 and LAT2.Int J Mol Sci. 2020 Oct 14;21(20):7573. doi: 10.3390/ijms21207573. Int J Mol Sci. 2020. PMID: 33066406 Free PMC article.
-
The human LAT1-4F2hc (SLC7A5-SLC3A2) transporter complex: Physiological and pathophysiological implications.Basic Clin Pharmacol Toxicol. 2023 Nov;133(5):459-472. doi: 10.1111/bcpt.13821. Epub 2022 Dec 13. Basic Clin Pharmacol Toxicol. 2023. PMID: 36460306 Free PMC article. Review.
-
Contribution of LAT1-4F2hc in Urological Cancers via Toll-like Receptor and Other Vital Pathways.Cancers (Basel). 2022 Jan 4;14(1):229. doi: 10.3390/cancers14010229. Cancers (Basel). 2022. PMID: 35008399 Free PMC article. Review.
References
-
- Betsunoh H., Fukuda T., Anzai N., Nishihara D., Mizuno T., Yuki H., et al. (2013). Increased expression of system large amino acid transporter (LAT)-1 mRNA is associated with invasive potential and unfavorable prognosis of human clear cell renal cell carcinoma. BMC Cancer 13, 509. 10.1186/1471-2407-13-509 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

