Vitamin D supplementation is effective for olanzapine-induced dyslipidemia
- PMID: 36895943
- PMCID: PMC9989177
- DOI: 10.3389/fphar.2023.1135516
Vitamin D supplementation is effective for olanzapine-induced dyslipidemia
Abstract
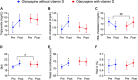
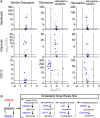
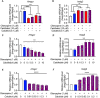
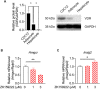
Olanzapine is an atypical antipsychotic drug that is clinically applied in patients with schizophrenia. It increases the risk of dyslipidemia, a disturbance of lipid metabolic homeostasis, usually characterized by increased low-density lipoprotein (LDL) cholesterol and triglycerides, and accompanied by decreased high-density lipoprotein (HDL) in the serum. In this study, analyzing the FDA Adverse Event Reporting System, JMDC insurance claims, and electronic medical records from Nihon University School of Medicine revealed that a co-treated drug, vitamin D, can reduce the incidence of olanzapine-induced dyslipidemia. In the following experimental validations of this hypothesis, short-term oral olanzapine administration in mice caused a simultaneous increase and decrease in the levels of LDL and HDL cholesterol, respectively, while the triglyceride level remained unaffected. Cholecalciferol supplementation attenuated these deteriorations in blood lipid profiles. RNA-seq analysis was conducted on three cell types that are closely related to maintaining cholesterol metabolic balance (hepatocytes, adipocytes, and C2C12) to verify the direct effects of olanzapine and the functional metabolites of cholecalciferol (calcifediol and calcitriol). Consequently, the expression of cholesterol-biosynthesis-related genes was reduced in calcifediol- and calcitriol-treated C2C12 cells, which was likely to be mediated by activating the vitamin D receptor that subsequently inhibited the cholesterol biosynthesis process via insulin-induced gene 2 regulation. This clinical big-data-based drug repurposing approach is effective in finding a novel treatment with high clinical predictability and a well-defined molecular mechanism.
Keywords: C2C12 cells; clinical big data; dyslipidemia; high-density lipoprotein; low-density lipoprotein; olanzapine; vitamin D.
Copyright © 2023 Zhou, Nagashima, Toda, Kobayashi, Suzuki, Nagayasu, Shirakawa, Asai and Kaneko.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

