Cutaneous Malignant Melanoma Presenting as an Isolated Splenic Metastasis: An Update
- PMID: 36895999
- PMCID: PMC9990732
- DOI: 10.14740/wjon1567
Cutaneous Malignant Melanoma Presenting as an Isolated Splenic Metastasis: An Update
Abstract
Although the spleen is a highly vascularized organ, metastatic deposits from non-hematolymphoid solid malignancies are rare. This is reasoned to the inherent resistance of the splenic parenchyma to harbor metastases. The splenic capsule, lack of afferent lymphatics, contractile properties of the spleen, and the angular and gyroid course of the splenic artery form several barriers against the metastatic spread of malignant tumors. Moreover, the immune cells in the white and red pulps of the spleen have strong defensive ability against the tumor cells. Metastasis from solid tumors to the spleen often occurs only during widespread distant spread. Malignant melanoma is a rare but fatal malignancy. Isolated splenic metastasis from malignant melanoma is exceptionally rare. Studies that addressed the splenic metastasis from cutaneous malignant melanoma are scarce. This minireview was performed to address this subject. Here we present an overview of the clinicopathologic features of isolated splenic metastatic melanoma. The diagnostic biochemical markers in melanoma are also discussed.
Keywords: Isolated; Melanoma; Metastasis; Single mass; Skin; Spleen.
Copyright 2023, Alshehri et al.
Conflict of interest statement
None to declare.
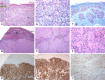
Figures
References
-
- Bouquet C, Frau E, Opolon P, Connault E, Abitbol M, Griscelli F, Yeh P. et al. Systemic administration of a recombinant adenovirus encoding a HSA-Angiostatin kringle 1-3 conjugate inhibits MDA-MB-231 tumor growth and metastasis in a transgenic model of spontaneous eye cancer. Mol Ther. 2003;7(2):174–184. doi: 10.1016/S1525-0016(02)00057-6. - DOI - PubMed
-
- Imada H, Nakata H, Horie A. [Radiological diagnosis of splenic metastasis and its prevalence at autopsy] Nihon Igaku Hoshasen Gakkai Zasshi. 1991;51(5):498–503. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
