The circadian control of tryptophan metabolism regulates the host response to pulmonary fungal infections
- PMID: 36896128
- PMCID: PMC9991457
- DOI: 10.1093/pnasnexus/pgad036
The circadian control of tryptophan metabolism regulates the host response to pulmonary fungal infections
Abstract
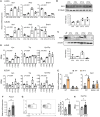
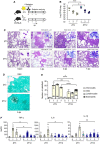
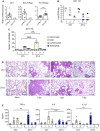
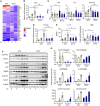
The environmental light/dark cycle has left its mark on the body's physiological functions to condition not only our inner biology, but also the interaction with external cues. In this scenario, the circadian regulation of the immune response has emerged as a critical factor in defining the host-pathogen interaction and the identification of the underlying circuitry represents a prerequisite for the development of circadian-based therapeutic strategies. The possibility to track down the circadian regulation of the immune response to a metabolic pathway would represent a unique opportunity in this direction. Herein, we show that the metabolism of the essential amino acid tryptophan, involved in the regulation of fundamental processes in mammals, is regulated in a circadian manner in both murine and human cells and in mouse tissues. By resorting to a murine model of pulmonary infection with the opportunistic fungus Aspergillus fumigatus, we showed that the circadian oscillation in the lung of the tryptophan-degrading enzyme indoleamine 2,3-dioxygenase (IDO)1, generating the immunoregulatory kynurenine, resulted in diurnal changes in the immune response and the outcome of fungal infection. In addition, the circadian regulation of IDO1 drives such diurnal changes in a pre-clinical model of cystic fibrosis (CF), an autosomal recessive disease characterized by progressive lung function decline and recurrent infections, thus acquiring considerable clinical relevance. Our results demonstrate that the circadian rhythm at the intersection between metabolism and immune response underlies the diurnal changes in host-fungal interaction, thus paving the way for a circadian-based antimicrobial therapy.
Keywords: Aspergillus fumigatus; IDO1; circadian rhythm; cystic fibrosis; kynurenines.
© The Author(s) 2023. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures

Similar articles
-
Th17/Treg imbalance in murine cystic fibrosis is linked to indoleamine 2,3-dioxygenase deficiency but corrected by kynurenines.Am J Respir Crit Care Med. 2013 Mar 15;187(6):609-20. doi: 10.1164/rccm.201207-1346OC. Epub 2013 Jan 10. Am J Respir Crit Care Med. 2013. PMID: 23306541
-
A Multifaceted Role of Tryptophan Metabolism and Indoleamine 2,3-Dioxygenase Activity in Aspergillus fumigatus-Host Interactions.Front Immunol. 2018 Jan 22;8:1996. doi: 10.3389/fimmu.2017.01996. eCollection 2017. Front Immunol. 2018. PMID: 29403477 Free PMC article. Review.
-
Genetic Polymorphisms Affecting IDO1 or IDO2 Activity Differently Associate With Aspergillosis in Humans.Front Immunol. 2019 May 7;10:890. doi: 10.3389/fimmu.2019.00890. eCollection 2019. Front Immunol. 2019. PMID: 31134053 Free PMC article.
-
Deletion of TDO2, IDO-1 and IDO-2 differentially affects mouse behavior and cognitive function.Behav Brain Res. 2016 Oct 1;312:102-17. doi: 10.1016/j.bbr.2016.06.018. Epub 2016 Jun 15. Behav Brain Res. 2016. PMID: 27316339
-
Indoleamine 2,3-dioxygenase 1 (IDO1): an up-to-date overview of an eclectic immunoregulatory enzyme.FEBS J. 2022 Oct;289(20):6099-6118. doi: 10.1111/febs.16086. Epub 2021 Jun 30. FEBS J. 2022. PMID: 34145969 Free PMC article. Review.
Cited by
-
Circadian metabolic adaptations to infections.Philos Trans R Soc Lond B Biol Sci. 2025 Jan 23;380(1918):20230473. doi: 10.1098/rstb.2023.0473. Epub 2025 Jan 23. Philos Trans R Soc Lond B Biol Sci. 2025. PMID: 39842481 Review.
-
Crosstalk between circadian clocks and pathogen niche.PLoS Pathog. 2024 May 9;20(5):e1012157. doi: 10.1371/journal.ppat.1012157. eCollection 2024 May. PLoS Pathog. 2024. PMID: 38723104 Free PMC article. Review.

