Quadratic Spin-Orbit Mechanism of the Electronic g-Tensor
- PMID: 36896579
- PMCID: PMC10061661
- DOI: 10.1021/acs.jctc.2c01213
Quadratic Spin-Orbit Mechanism of the Electronic g-Tensor
Abstract
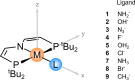
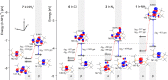
Understanding how the electronic g-tensor is linked to the electronic structure is desirable for the correct interpretation of electron paramagnetic resonance spectra. For heavy-element compounds with large spin-orbit (SO) effects, this is still not completely clear. We report our investigation of quadratic SO contributions to the g-shift in heavy transition metal complexes. We implemented third-order perturbation theory in order to analyze the contributions arising from frontier molecular spin orbitals (MSOs). We show that the dominant quadratic SO term─spin-Zeeman (SO2/SZ)─generally makes a negative contribution to the g-shift, irrespective of the particular electronic configuration or molecular symmetry. We further analyze how the SO2/SZ contribution adds to or subtracts from the linear orbital-Zeeman (SO/OZ) contribution to the individual principal components of the g-tensor. Our study suggests that the SO2/SZ mechanism decreases the anisotropy of the g-tensor in early transition metal complexes and increases it in late transition metal complexes. Finally, we apply MSO analysis to the investigation of g-tensor trends in a set of closely related Ir and Rh pincer complexes and evaluate the influence of different chemical factors (the nuclear charge of the central atom and the terminal ligand) on the magnitudes of the g-shifts. We expect our conclusions to aid the understanding of spectra in magnetic resonance investigations of heavy transition metal compounds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Mabbs F. E.; Collison D.. Electron Paramagnetic Resonance of d Transition Metal Compounds, 1st ed.; Elsevier Science: Amsterdam, 2013.
-
- Abragam A.; Bleaney B.. Electron Paramagnetic Resonance of Transition Ions. Oxford Classic Texts in the Physical Sciences; Oxford University Press: Oxford, New York, 2012.
-
- Chibotaru L. F.Ab Initio Methodology for Pseudospin Hamiltonians of Anisotropic Magnetic Complexes. Advances in Chemical Physics; John Wiley & Sons, Ltd, 2013; pp 397–519.
LinkOut - more resources
Full Text Sources

