High spatial overlap but diverging age-related trajectories of cortical magnetic resonance imaging markers aiming to represent intracortical myelin and microstructure
- PMID: 36896711
- PMCID: PMC10171508
- DOI: 10.1002/hbm.26259
High spatial overlap but diverging age-related trajectories of cortical magnetic resonance imaging markers aiming to represent intracortical myelin and microstructure
Abstract
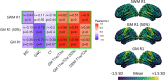
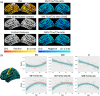
Statistical effects of cortical metrics derived from standard T1- and T2-weighted magnetic resonance imaging (MRI) images, such as gray-white matter contrast (GWC), boundary sharpness coefficient (BSC), T1-weighted/T2-weighted ratio (T1w/T2w), and cortical thickness (CT), are often interpreted as representing or being influenced by intracortical myelin content with little empirical evidence to justify these interpretations. We first examined spatial correspondence with more biologically specific microstructural measures, and second compared between-marker age-related trends with the underlying hypothesis that different measures primarily driven by similar changes in myelo- and microstructural underpinnings should be highly related. Cortical MRI markers were derived from MRI images of 127 healthy subjects, aged 18-81, using cortical surfaces that were generated with the CIVET 2.1.0 pipeline. Their gross spatial distributions were compared with gene expression-derived cell-type densities, histology-derived cytoarchitecture, and quantitative R1 maps acquired on a subset of participants. We then compared between-marker age-related trends in their shape, direction, and spatial distribution of the linear age effect. The gross anatomical distributions of cortical MRI markers were, in general, more related to myelin and glial cells than neuronal indicators. Comparing MRI markers, our results revealed generally high overlap in spatial distribution (i.e., group means), but mostly divergent age trajectories in the shape, direction, and spatial distribution of the linear age effect. We conclude that the microstructural properties at the source of spatial distributions of MRI cortical markers can be different from microstructural changes that affect these markers in aging.
Keywords: T1-weighted/T2-weighted ratio; aging; boundary sharpness coefficient; cortical myelin; cortical thickness; gray-white matter contrast; magnetic resonance imaging.
© 2023 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors have no actual or potential conflicts of interest.
Figures







References
-
- Alexander‐Bloch, A. F. , Shou, H. , Liu, S. , Satterthwaite, T. D. , Glahn, D. C. , Shinohara, R. T. , Vandekar, S. N. , & Raznahan, A. (2018). On testing for spatial correspondence between maps of human brain structure and function. NeuroImage, 178, 540–551. 10.1016/j.neuroimage.2018.05.070 - DOI - PMC - PubMed
-
- Amunts, K. , Lepage, C. , Borgeat, L. , Mohlberg, H. , Dickscheid, T. , Rousseau, M.‐É. , Bludau, S. , Bazin, P.‐L. , Lewis, L. B. , Oros‐Peusquens, A.‐M. , Shah, N. J. , Lippert, T. , Zilles, K. , & Evans, A. C. (2013). BigBrain: An ultrahigh‐resolution 3D human brain model. Science, 340(6139), 1472–1475. 10.1126/science.1235381 - DOI - PubMed
-
- Arevalo‐Rodriguez, I. , Smailagic, N. , Roqué, I. , Figuls, M. , Ciapponi, A. , Sanchez‐Perez, E. , Giannakou, A. , Pedraza, O. L. , Bonfill Cosp, X. , & Cullum, S. (2015). Mini‐mental state examination (MMSE) for the detection of Alzheimer's disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews, 2015, CD010783. 10.1002/14651858.cd010783.pub2 - DOI - PMC - PubMed
-
- Bedford, S. A. , Park, M. T. M. , Devenyi, G. A. , Tullo, S. , Germann, J. , Patel, R. , Anagnostou, E. , Baron‐Cohen, S. , Bullmore, E. T. , Chura, L. R. , Craig, M. C. , Ecker, C. , Floris, D. L. , Holt, R. J. , Lenroot, R. , Lerch, J. P. , Lombardo, M. V. , Murphy, D. G. M. , Raznahan, A. , … Chakravarty, M. M. (2019). Large‐scale analyses of the relationship between sex, age and intelligence quotient heterogeneity and cortical morphometry in autism spectrum disorder. Molecular Psychiatry, 25, 614–628. 10.1038/s41380-019-0420-6 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

