Phosphorylation of KRT8 (keratin 8) by excessive mechanical load-activated PKN (protein kinase N) impairs autophagosome initiation and contributes to disc degeneration
- PMID: 36897022
- PMCID: PMC10392755
- DOI: 10.1080/15548627.2023.2186099
Phosphorylation of KRT8 (keratin 8) by excessive mechanical load-activated PKN (protein kinase N) impairs autophagosome initiation and contributes to disc degeneration
Abstract
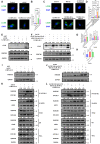
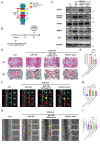
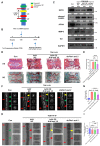
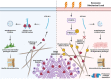
Excessive mechanical load (overloading) is a well-documented pathogenetic factor for many mechano stress-induced pathologies, i.e. intervertebral disc degeneration (IDD). Under overloading, the balance between anabolism and catabolism within nucleus pulposus (NP) cells are badly thrown off, and NP cells undergo apoptosis. However, little is known about how the overloading is transduced to the NP cells and contributes to disc degeneration. The current study shows that conditional knockout of Krt8 (keratin 8) within NP aggravates load-induced IDD in vivo, and overexpression of Krt8 endows NP cells greater resistance to overloading-induced apoptosis and degeneration in vitro. Discovery-driven experiments shows that phosphorylation of KRT8 on Ser43 by overloading activated RHOA-PKN (protein kinase N) impedes trafficking of Golgi resident small GTPase RAB33B, suppresses the autophagosome initiation and contributes to IDD. Overexpression of Krt8 and knockdown of Pkn1 and Pkn2, at an early stage of IDD, ameliorates disc degeneration; yet only knockdown of Pkn1 and Pkn2, when treated at late stage of IDD, shows a therapeutic effect. This study validates a protective role of Krt8 during overloading-induced IDD and demonstrates that targeting overloading activation of PKNs could be a novel and effective approach to mechano stress-induced pathologies with a wider window of therapeutic opportunity.Abbreviations: AAV: adeno-associated virus; AF: anulus fibrosus; ANOVA: analysis of variance; ATG: autophagy related; BSA: bovine serum albumin; cDNA: complementary deoxyribonucleic acid; CEP: cartilaginous endplates; CHX: cycloheximide; cKO: conditional knockout; Cor: coronal plane; CT: computed tomography; Cy: coccygeal vertebra; D: aspartic acid; DEG: differentially expressed gene; DHI: disc height index; DIBA: dot immunobinding assay; dUTP: 2'-deoxyuridine 5'-triphosphate; ECM: extracellular matrix; EDTA: ethylene diamine tetraacetic acid; ER: endoplasmic reticulum; FBS: fetal bovine serum; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GPS: group-based prediction system; GSEA: gene set enrichment analysis; GTP: guanosine triphosphate; HE: hematoxylin-eosin; HRP: horseradish peroxidase; IDD: intervertebral disc degeneration; IF: immunofluorescence staining; IL1: interleukin 1; IVD: intervertebral disc; KEGG: Kyoto encyclopedia of genes and genomes; KRT8: keratin 8; KD: knockdown; KO: knockout; L: lumbar vertebra; LBP: low back pain; LC/MS: liquid chromatograph mass spectrometer; LSI: mouse lumbar instability model; MAP1LC3/LC3: microtubule associated protein 1 light chain 3; MMP3: matrix metallopeptidase 3; MRI: nuclear magnetic resonance imaging; NC: negative control; NP: nucleus pulposus; PBS: phosphate-buffered saline; PE: p-phycoerythrin; PFA: paraformaldehyde; PI: propidium iodide; PKN: protein kinase N; OE: overexpression; PTM: post translational modification; PVDF: polyvinylidene fluoride; qPCR: quantitative reverse-transcriptase polymerase chain reaction; RHOA: ras homolog family member A; RIPA: radio immunoprecipitation assay; RNA: ribonucleic acid; ROS: reactive oxygen species; RT: room temperature; TCM: rat tail compression-induced IDD model; TCS: mouse tail suturing compressive model; S: serine; Sag: sagittal plane; SD rats: Sprague-Dawley rats; shRNA: short hairpin RNA; siRNA: small interfering RNA; SOFG: safranin O-fast green; SQSTM1: sequestosome 1; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling; VG/ml: viral genomes per milliliter; WCL: whole cell lysate.
Keywords: Autophagosome initiation; Protein kinase N; intervertebral disc degeneration; keratin 8; phosphorylation.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures



References
-
- Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:968–974. - PubMed
-
- Patrick N, Emanski E, Knaub MA.. Acute and chronic low back pain. Med Clin North Am. 2014;98:777-89, xii. - PubMed
-
- Knezevic NN, Candido KD, Vlaeyen JWS, et al. Low back pain. Lancet. 2021;398:78–92. - PubMed
-
- de Schepper EI, Damen J, van Meurs JB, et al. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine (Phila Pa 1976). 2010;35:531–536. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
