Efficacy, durability, and safety of faricimab with extended dosing up to every 16 weeks in Japanese patients with diabetic macular edema: 1-year results from the Japan subgroup of the phase 3 YOSEMITE trial
- PMID: 36897413
- PMCID: PMC9999055
- DOI: 10.1007/s10384-023-00979-8
Efficacy, durability, and safety of faricimab with extended dosing up to every 16 weeks in Japanese patients with diabetic macular edema: 1-year results from the Japan subgroup of the phase 3 YOSEMITE trial
Abstract
Purpose: To evaluate efficacy, durability, and safety of faricimab in Japanese patients with diabetic macular edema (DME).
Study design: Subgroup analysis of 2 global, multicenter, randomized, double-masked, active-comparator-controlled, phase 3 trials (YOSEMITE, NCT03622580; RHINE, NCT03622593).
Methods: Patients with DME were randomized 1:1:1 to intravitreal faricimab 6.0 mg every 8 weeks (Q8W), faricimab 6.0 mg per personalized treatment interval (PTI), or aflibercept 2.0 mg Q8W through week 100. Primary endpoint was best-corrected visual acuity (BCVA) change from baseline at 1 year, averaged over weeks 48, 52, and 56. This is the first time 1-year outcomes between Japanese patients (only enrolled into YOSEMITE) and the pooled YOSEMITE/RHINE cohort (N = 1891) have been compared.
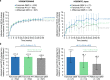
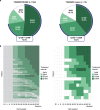
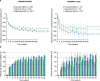
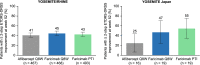
Results: The YOSEMITE Japan subgroup included 60 patients randomized to faricimab Q8W (n = 21), faricimab PTI (n = 19), or aflibercept Q8W (n = 20). Consistent with global results, the adjusted mean (95.04% confidence interval) BCVA change at 1 year in the Japan subgroup was comparable with faricimab Q8W (+11.1 [7.6-14.6] letters), faricimab PTI (+8.1 [4.4-11.7] letters), and aflibercept Q8W (+6.9 [3.3-10.5] letters). At week 52, 13 (72%) patients in the faricimab PTI arm achieved ≥ Q12W dosing, including 7 (39%) patients receiving Q16W dosing. Anatomic improvements with faricimab were generally consistent between the Japan subgroup and pooled YOSEMITE/RHINE cohort. Faricimab was well tolerated; no new or unexpected safety signals were identified.
Conclusion: Consistent with global results, faricimab up to Q16W offered durable vision gains and improved anatomic and disease-specific outcomes among Japanese patients with DME.
Keywords: Angiopoietin-2; Anti-VEGF therapy; Diabetic macular edema; Faricimab; Vascular stability.
© 2023. Japanese Ophthalmological Society.
Conflict of interest statement
M. Shimura, Consulting fees (Chugai, Roche), Lecture fees (Chugai); S. Kitano, Consulting fees (Chugai), Speaker fees (Bayer, Novartis); N. Ogata, None; Y. Mitamura, Grants (Santen), Speaker fee (Bayer, Chugai, Novartis, Santen); H. Oh, Grants (Chugai), Lecture fees (Alcon, Bayer, Kowa, Novartis, Santen); H. Ochi, Employee (Chugai); S. Ohsawa, Employee (Chugai); A. Hirakata, None.
Figures

References
-
- Morizane Y, Morimoto N, Fujiwara A, Kawasaki R, Yamashita H, Ogura Y, et al. Incidence and causes of visual impairment in Japan: the first nation-wide complete enumeration survey of newly certified visually impaired individuals. Jpn J Ophthalmol. 2019;63:26–33. doi: 10.1007/s10384-018-0623-4. - DOI - PubMed

