Constitutive expression and distinct properties of IFN-epsilon protect the female reproductive tract from Zika virus infection
- PMID: 36897927
- PMCID: PMC10032502
- DOI: 10.1371/journal.ppat.1010843
Constitutive expression and distinct properties of IFN-epsilon protect the female reproductive tract from Zika virus infection
Abstract
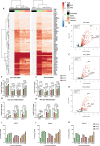
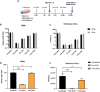
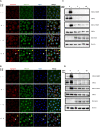
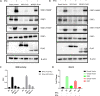
The immunological surveillance factors controlling vulnerability of the female reproductive tract (FRT) to sexually transmitted viral infections are not well understood. Interferon-epsilon (IFNɛ) is a distinct, immunoregulatory type-I IFN that is constitutively expressed by FRT epithelium and is not induced by pathogens like other antiviral IFNs α, β and λ. We show the necessity of IFNɛ for Zika Virus (ZIKV) protection by: increased susceptibility of IFNɛ-/- mice; their "rescue" by intravaginal recombinant IFNɛ treatment and blockade of protective endogenous IFNɛ by neutralising antibody. Complementary studies in human FRT cell lines showed IFNɛ had potent anti-ZIKV activity, associated with transcriptome responses similar to IFNλ but lacking the proinflammatory gene signature of IFNα. IFNɛ activated STAT1/2 pathways similar to IFNα and λ that were inhibited by ZIKV-encoded non-structural (NS) proteins, but not if IFNε exposure preceded infection. This scenario is provided by the constitutive expression of endogenous IFNε. However, the IFNɛ expression was not inhibited by ZIKV NS proteins despite their ability to antagonise the expression of IFNβ or λ. Thus, the constitutive expression of IFNɛ provides cellular resistance to viral strategies of antagonism and maximises the antiviral activity of the FRT. These results show that the unique spatiotemporal properties of IFNε provides an innate immune surveillance network in the FRT that is a significant barrier to viral infection with important implications for prevention and therapy.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Pomar L, Lambert V, Madec Y, Vouga M, Pomar C, Matheus S, et al. Placental infection by Zika virus in French Guiana. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2019. Epub 2019/11/28. doi: 10.1002/uog.21936 . - DOI - PubMed
-
- Rice ME, Galang RR, Roth NM, Ellington SR, Moore CA, Valencia-Prado M, et al. Vital Signs: Zika-Associated Birth Defects and Neurodevelopmental Abnormalities Possibly Associated with Congenital Zika Virus Infection—U.S. Territories and Freely Associated States, 2018. MMWR Morbidity and mortality weekly report. 2018;67(31):858–67. Epub 2018/08/10. doi: 10.15585/mmwr.mm6731e1 ; PubMed Central PMCID: PMC6089332. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

