An XBP1s-PIM-2 positive feedback loop controls IL-15-mediated survival of natural killer cells
- PMID: 36897958
- PMCID: PMC10216801
- DOI: 10.1126/sciimmunol.abn7993
An XBP1s-PIM-2 positive feedback loop controls IL-15-mediated survival of natural killer cells
Abstract
Spliced X-box-binding protein 1 (XBP1s) is an essential transcription factor downstream of interleukin-15 (IL-15) and AKT signaling, which controls cell survival and effector functions of human natural killer (NK) cells. However, the precise mechanisms, especially the downstream targets of XBP1s, remain unknown. In this study, by using XBP1 conditional knockout mice, we found that XBP1s is critical for IL-15-mediated NK cell survival but not proliferation in vitro and in vivo. Mechanistically, XBP1s regulates homeostatic NK cell survival by targeting PIM-2, a critical anti-apoptotic gene, which in turn stabilizes XBP1s protein by phosphorylating it at Thr58. In addition, XBP1s enhances the effector functions and antitumor immunity of NK cells by recruiting T-bet to the promoter region of Ifng. Collectively, our findings identify a previously unknown mechanism by which IL-15-XBP1s signaling regulates the survival and effector functions of NK cells.
Figures
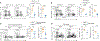
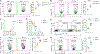

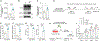

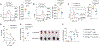

References
-
- Spits H, Bernink JH, Lanier L, NK cells and type 1 innate lymphoid cells: Partners in host defense. Nat. Immunol 17, 758–764 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

