Phase II Clinical Trial of Axitinib and Avelumab in Patients With Recurrent/Metastatic Adenoid Cystic Carcinoma
- PMID: 36898078
- PMCID: PMC10414730
- DOI: 10.1200/JCO.22.02221
Phase II Clinical Trial of Axitinib and Avelumab in Patients With Recurrent/Metastatic Adenoid Cystic Carcinoma
Abstract
Purpose: We conducted a phase II trial evaluating the efficacy of VEGFR inhibitor axitinib and PD-L1 inhibitor avelumab in patients with recurrent/metastatic adenoid cystic carcinoma (R/M ACC).
Patients and methods: Eligible patients had R/M ACC with progression within 6 months before enrollment. Treatment consisted of axitinib and avelumab. The primary end point was objective response rate (ORR) per RECIST 1.1; secondary end points included progression-free survival (PFS), overall survival (OS), and toxicity. Simon's optimal two-stage design tested the null hypothesis of ORR ≤5% versus ORR ≥20% at 6 months; ≥4 responses in 29 patients would reject the null hypothesis.
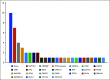
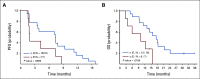
Results: Forty patients enrolled from July 2019 to June 2021; 28 were evaluable for efficacy (six screen failures; six evaluable for safety only). The confirmed ORR was 18% (95% CI, 6.1 to 36.9); there was one unconfirmed partial response (PR). Two patients achieved PR after 6 months; thus, the ORR at 6 months was 14%. The median follow-up time for surviving patients was 22 months (95% CI, 16.6 to 39.1 months). The median PFS was 7.3 months (95% CI, 3.7 to 11.2 months), 6-month PFS rate was 57% (95% CI, 41 to 78), and median OS was 16.6 months (95% CI, 12.4 to not reached months). Most common treatment-related adverse events (TRAEs) included fatigue (62%), hypertension (32%), and diarrhea (32%). Ten (29%) patients had serious TRAEs, all grade 3; four patients (12%) discontinued avelumab, and nine patients (26%) underwent axitinib dose reduction.
Conclusion: The study reached its primary end point with ≥4 PRs in 28 evaluable patients (confirmed ORR of 18%). The potential added benefit of avelumab to axitinib in ACC requires further investigation.
Trial registration: ClinicalTrials.gov NCT03990571.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures



References
-
- Ferrarotto R, Heymach JV, Glisson BS: MYB-fusions and other potential actionable targets in adenoid cystic carcinoma. Curr Opin Oncol 28:195-200, 2016 - PubMed
-
- Laurie SA, Ho AL, Fury MG, et al. : Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: A systematic review. Lancet Oncol 12:815-824, 2011 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

