How I treat erythropoietic protoporphyria and X-linked protoporphyria
- PMID: 36898083
- PMCID: PMC10646811
- DOI: 10.1182/blood.2022018688
How I treat erythropoietic protoporphyria and X-linked protoporphyria
Abstract
Erythropoietic protoporphyria (EPP) is an inherited cutaneous porphyria caused by reduced expression of ferrochelatase, the enzyme that catalyzes the final step in heme biosynthesis. The resultant accumulation of protoporphyrin IX leads to severe, painful cutaneous photosensitivity, as well as potentially life-threatening liver disease in a small percentage of patients. X-linked protoporphyria (XLP) is clinically similar to EPP but results from increased activity of δ-aminolevulinic acid synthase 2, the first step in heme biosynthesis in the bone marrow, and also causes protoporphyrin accumulation. Although historically the management of EPP and XLP (collectively termed protoporphyria) centered around avoidance of sunlight, novel therapies have recently been approved or are in development, which will alter the therapeutic landscape for these conditions. We present 3 patient cases, highlighting key treatment considerations in patients with protoporphyria, including (1) approach to photosensitivity, (2) managing iron deficiency in protoporphyria, and (3) understanding hepatic failure in protoporphyria.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.K.L. serves as a consultant for Mitsubishi Tanabe, Alnylam Pharmaceuticals, and Recordati Pharmaceuticals. A.K.D. has received research funding from Disc Medicine for other research unrelated to this publication, and serves as a consultant for Alnylam Pharmaceuticals and Recordati Pharmaceuticals.
Figures
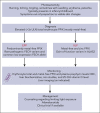


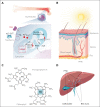
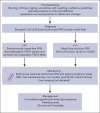
References
-
- Bissell DM, Anderson KE, Bonkovsky HL. Porphyria. N Engl J Med. 2017;377(9):862–872. - PubMed
-
- Balwani M, Bloomer R, Desnick R, Porphyrias Consortium of the NIH-Sponsored Rare Diseases Clinical Research Network . In: GeneReviews. Adam MP, Mirzaa GM, Pagon RA, editors. University of Seattle; 2017. Erythropoietic protoporphyria, autosomal recessive; pp. 1993–2023.
-
- Elder G, Harper P, Badminton M, Sandberg S, Deybach JC. The incidence of inherited porphyrias in Europe. J Inherit Metab Dis. 2013;36(5):849–857. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

