Sonochemical advanced oxidation process for the degradation of furosemide in water: Effects of sonication's conditions and scavengers
- PMID: 36898249
- PMCID: PMC10020096
- DOI: 10.1016/j.ultsonch.2023.106361
Sonochemical advanced oxidation process for the degradation of furosemide in water: Effects of sonication's conditions and scavengers
Abstract
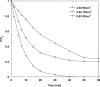
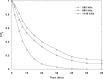
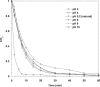
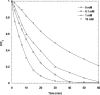
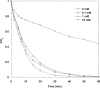
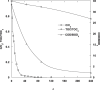
The intensive consumption of pharmaceuticals and drugs in the last decades has led to their increased concentrations in wastewaters from industrial sources. The present paper deals, for the first time, with the sonochemical degradation and mineralization of furosemide (FSM) in water. FSM is a potent loop diuretic used to treat fluid build-up due to heart failure, liver scarring, or kidney disease. The influence of several operating parameters such as acoustic intensity, ultrasonic frequency, initial FSM concentration, solution's pH, nature of the dissolved gas (Ar, air and N2) and radical scavengers (2-propanol and tert-butanol) on the oxidation of FSM was assessed. The obtained results showed that the degradation rate of the drug increased significantly with the increase of the acoustic intensity in the range of 0.83 to 4.3 W cm-2 and decreased with the augmentation of the frequency in the range of 585-1140 kHz. It was also found that the initial rate of the sonolytic degradation of FSM increased with the increase of its initial concentration (2, 5, 10, 15 and 20 mg/L). The most significant degradation was achieved in acidic conditions at pH 2, while in terms of saturating gas, the rate of FSM degradation decreased in the order of Ar > air > N2. The FSM degradation experiments with radical scavengers showed that the diuretic molecule degraded mainly at the interfacial region of the bubble by hydroxyl radical attack. Additionally, in terms of acoustic conditions, the sono-degradation of 30.24 µmol L-1 of FSM solution demonstrate an optimal performance at 585 kHz and 4.3 W/cm2, the results indicated that even if the ultrasonic action eliminated the total concentration of FSM within 60 min, a low degree of mineralization was obtained due to the by-products formed during the sono-oxidation process. The ultrasonic process transforms FSM into biodegradable and environmentally friendly organic by-products that could be treated in a subsequent biological treatment. Besides, the efficiency of the sonolytic degradation of FSM in real environmental matrices such as natural mineral water and seawater was demonstrated. Consequently, the sonochemical advanced oxidation process represent a very interesting technique for the treatment of water contaminated with FSM.
Keywords: Acoustic intensity; Degradation; Furosemide; Scavengers; Ultrasonic frequency; Water matrix.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Gomes I.B., Simões L.C., Simões M. The effects of emerging environmental contaminants on Stenotrophomonas maltophilia isolated from drinking water in planktonic and sessile states. Sci. Total Environ. 2018;643:1348–1356. - PubMed
-
- Heberer T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment : a review of recent research data. Toxicol. Lett. 2002;131:5–17. - PubMed
-
- Bradley P.M., Journey C.A., Romanok K.M., Barber L.B., Buxton H.T., Foreman W.T., Furlong E.T., Glassmeyer S.T., Hladik M.L., Iwanowicz L.R., Jones D.K., Kolpin D.W., Kuivila K.M., Loftin K.A., Mills M.A., Meyer M.T., Orlando J.L., Reilly T.J., Smalling K.L., Villeneuve D.L. Expanded target-chemical analysis reveals extensive mixed-organic-contaminant exposure in U.S. Streams. Environ. Sci. Technol. 2017;51(9):4792–4802. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

