Anti-VEGF therapy improves EGFR-vIII-CAR-T cell delivery and efficacy in syngeneic glioblastoma models in mice
- PMID: 36898734
- PMCID: PMC10008211
- DOI: 10.1136/jitc-2022-005583
Anti-VEGF therapy improves EGFR-vIII-CAR-T cell delivery and efficacy in syngeneic glioblastoma models in mice
Abstract
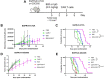
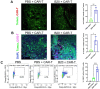
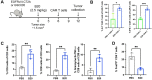
Chimeric antigen receptor (CAR)-T cells have revolutionized the treatment of multiple types of hematological malignancies, but have shown limited efficacy in patients with glioblastoma (GBM) or other solid tumors. This may be largely due to the immunosuppressive tumor microenvironment (TME) that compromises CAR-T cells' delivery and antitumor activity. We previously showed that blocking vascular endothelial growth factor (VEGF) signaling can normalize tumor vessels in murine and human tumors, including GBM, breast, liver, and rectal carcinomas. Moreover, we demonstrated that vascular normalization can improve the delivery of CD8+ T cells and the efficacy of immunotherapy in breast cancer models in mice. In fact, the US FDA (Food and drug administration) has approved seven different combinations of anti-VEGF drugs and immune checkpoint blockers for liver, kidney, lung and endometrial cancers in the past 3 years. Here, we tested the hypothesis that anti-VEGF therapy can improve the delivery and efficacy of CAR-T cells in immunocompetent mice bearing orthotopic GBM tumors. We engineered two syngeneic mouse GBM cell lines (CT2A and GSC005) to express EGFRvIII-one of the most common neoantigens in human GBM-and CAR T cells to recognize EGFRvIII. We found that treatment with the anti-mouse VEGF antibody (B20) improved CAR-T cell infiltration and distribution throughout the GBM TME, delayed tumor growth, and prolonged survival of GBM-bearing mice compared with EGFRvIII-CAR-T cell therapy alone. Our findings provide compelling data and a rationale for clinical evaluation of anti-VEGF agents with CAR T cells for GBM patients.
Keywords: tumor microenvironment.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RKJ received consultant fees from Bristol Myers Squibb, Elpis, Innocoll, SPARC, and SynDevRx; owns equity in Accurius, Enlight, and SynDevRx; is on the Board of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, and Tekla World Healthcare Fund; and received a research grant from Boehringer Ingelheim. JR owns stock options in Dynamic Cell Therapies.
Figures
References
-
- Young RM, Engel NW, Uslu U, et al. . Next-Generation CAR T-cell therapies. Cancer Discov 2022;12:1625–33. 10.1158/2159-8290.CD-21-1683 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
