Women's experiences with using patient-reported outcome and experience measures in routine perinatal care in the Netherlands: a mixed-methods study
- PMID: 36898740
- PMCID: PMC10008176
- DOI: 10.1136/bmjopen-2022-064452
Women's experiences with using patient-reported outcome and experience measures in routine perinatal care in the Netherlands: a mixed-methods study
Abstract
Objectives: To gain insight into the experiences of women with completing and discussing patient-reported outcome measures (PROM) and patient-reported experience measures (PREM), and tailoring their care based on their outcomes.
Design: A mixed-methods prospective cohort study.
Setting: Seven obstetric care networks in the Netherlands that implemented a set of patient-centred outcome measures for pregnancy and childbirth (PCB set), published by the International Consortium for Health Outcomes Measurement.
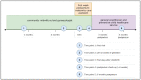
Participants: All women, receiving the PROM and PREM questionnaires as part of their routine perinatal care, received an invitation for a survey (n=460) and an interview (n=16). The results of the survey were analysed using descriptive statistics; thematic inductive content analysis was applied on the data from open text answers and the interviews.
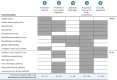
Results: More than half of the survey participants (n=255) felt the need to discuss the outcomes of PROM and PREM with their care professionals. The time spent on completing questionnaires and the comprehensiveness of the questions was scored 'good' by most of the survey participants. From the interviews, four main themes were identified: content of the PROM and PREM questionnaires, application of these outcomes in perinatal care, discussing PREM and data capture tool. Important facilitators included awareness of health status, receiving personalised care based on their outcomes and the relevance of discussing PREM 6 months post partum. Barriers were found in insufficient information about the goal of PROM and PREM for individual care, technical problems in data capture tools and discrepancy between the questionnaire topics and the care pathway.
Conclusions: This study showed that women found the PCB set an acceptable and useful instrument for symptom detection and personalised care up until 6 months post partum. This patient evaluation of the PCB set has several implications for practice regarding the questionnaire content, role of care professionals and congruity with care pathways.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AF and ML-dR were chair and member respectively of the ICHOM working group that developed the PCB standard outcome set. The other authors have nothing to declare.
Figures
References
-
- Porter ME, Teisberg EO. Redefining health care: creating value-based competition on results. Harvard Business Press, 2006.
-
- Gliklich RD, Leavy M. Registries for evaluating patient outcomes: A user’s guide [Internet]. 2014. - PubMed
-
- U.S. Department of Health Services Human F.D.A. Center for Drug Evaluation Research, U.S. Department of Health Human Services F.D.A. Center for Biologics Evaluation Research, U.S. Department of Health Human Services F.D.A . Center for devices radiological health, guidance for industry: patient-reported outcome measures: use in medical product development to supportlabeling claims: draft guidance. health qual life outcomes. Health Qual Life Outcomes 2006;4:79. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
