Microglia activation in the presence of intact blood-brain barrier and disruption of hippocampal neurogenesis via IL-6 and IL-18 mediate early diffuse neuropsychiatric lupus
- PMID: 36898766
- PMCID: PMC10176423
- DOI: 10.1136/ard-2022-223506
Microglia activation in the presence of intact blood-brain barrier and disruption of hippocampal neurogenesis via IL-6 and IL-18 mediate early diffuse neuropsychiatric lupus
Abstract
Introduction: Inflammatory mediators are detected in the cerebrospinal fluid of systemic lupus erythematosus patients with central nervous system involvement (NPSLE), yet the underlying cellular and molecular mechanisms leading to neuropsychiatric disease remain elusive.
Methods: We performed a comprehensive phenotyping of NZB/W-F1 lupus-prone mice including tests for depression, anxiety and cognition. Immunofluorescence, flow cytometry, RNA-sequencing, qPCR, cytokine quantification and blood-brain barrier (BBB) permeability assays were applied in hippocampal tissue obtained in both prenephritic (3-month-old) and nephritic (6-month-old) lupus mice and matched control strains. Healthy adult hippocampal neural stem cells (hiNSCs) were exposed ex vivo to exogenous inflammatory cytokines to assess their effects on proliferation and apoptosis.
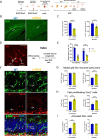
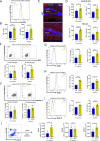
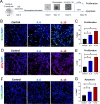
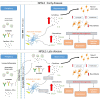
Results: At the prenephritic stage, BBB is intact yet mice exhibit hippocampus-related behavioural deficits recapitulating the human diffuse neuropsychiatric disease. This phenotype is accounted by disrupted hippocampal neurogenesis with hiNSCs exhibiting increased proliferation combined with decreased differentiation and increased apoptosis in combination with microglia activation and increased secretion of proinflammatory cytokines and chemokines. Among these cytokines, IL-6 and IL-18 directly induce apoptosis of adult hiNSCs ex vivo. During the nephritic stage, BBB becomes disrupted which facilitates immune components of peripheral blood, particularly B-cells, to penetrate into the hippocampus further augmenting inflammation with locally increased levels of IL-6, IL-12, IL-18 and IL-23. Of note, an interferon gene signature was observed only at nephritic-stage.
Conclusion: An intact BBB with microglial activation disrupting the formation of new neurons within the hippocampus represent early events in NPSLE. Disturbances of the BBB and interferon signature are evident later in the course of the disease.
Keywords: Autoimmunity; Cytokines; Lupus Erythematosus, Systemic.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Nikolopoulos D, Kostopoulou M, Pieta A, et al. . Evolving phenotype of systemic lupus erythematosus in Caucasians: low incidence of lupus nephritis, high burden of neuropsychiatric disease and increased rates of late-onset lupus in the “ Attikon ” cohort. Lupus 2020;29:514–22. 10.1177/0961203320908932 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

