Biomechanics and neural circuits for vestibular-induced fine postural control in larval zebrafish
- PMID: 36898983
- PMCID: PMC10006170
- DOI: 10.1038/s41467-023-36682-y
Biomechanics and neural circuits for vestibular-induced fine postural control in larval zebrafish
Abstract
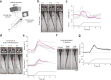
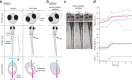
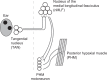
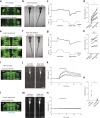
Land-walking vertebrates maintain a desirable posture by finely controlling muscles. It is unclear whether fish also finely control posture in the water. Here, we showed that larval zebrafish have fine posture control. When roll-tilted, fish recovered their upright posture using a reflex behavior, which was a slight body bend near the swim bladder. The vestibular-induced body bend produces a misalignment between gravity and buoyancy, generating a moment of force that recovers the upright posture. We identified the neural circuits for the reflex, including the vestibular nucleus (tangential nucleus) through reticulospinal neurons (neurons in the nucleus of the medial longitudinal fasciculus) to the spinal cord, and finally to the posterior hypaxial muscles, a special class of muscles near the swim bladder. These results suggest that fish maintain a dorsal-up posture by frequently performing the body bend reflex and demonstrate that the reticulospinal pathway plays a critical role in fine postural control.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Cerebellar Purkinje cells control posture in larval zebrafish (Danio rerio).Elife. 2025 Apr 24;13:RP97614. doi: 10.7554/eLife.97614. Elife. 2025. PMID: 40272244 Free PMC article.
-
Descending control of swim posture by a midbrain nucleus in zebrafish.Neuron. 2014 Aug 6;83(3):679-91. doi: 10.1016/j.neuron.2014.04.018. Epub 2014 Jul 24. Neuron. 2014. PMID: 25066082 Free PMC article.
-
The Vestibulospinal Nucleus Is a Locus of Balance Development.J Neurosci. 2024 Jul 24;44(30):e2315232024. doi: 10.1523/JNEUROSCI.2315-23.2024. J Neurosci. 2024. PMID: 38777599 Free PMC article.
-
Spinal and supraspinal postural networks.Brain Res Rev. 2008 Jan;57(1):212-21. doi: 10.1016/j.brainresrev.2007.06.017. Epub 2007 Jul 27. Brain Res Rev. 2008. PMID: 17822773 Free PMC article. Review.
-
Development of vestibular behaviors in zebrafish.Curr Opin Neurobiol. 2018 Dec;53:83-89. doi: 10.1016/j.conb.2018.06.004. Epub 2018 Jun 26. Curr Opin Neurobiol. 2018. PMID: 29957408 Free PMC article. Review.
Cited by
-
The mesencephalic locomotor region recruits V2a reticulospinal neurons to drive forward locomotion in larval zebrafish.Nat Neurosci. 2023 Oct;26(10):1775-1790. doi: 10.1038/s41593-023-01418-0. Epub 2023 Sep 4. Nat Neurosci. 2023. PMID: 37667039 Free PMC article.
-
Spatially ordered recruitment of fast muscles in accordance with movement strengths in larval zebrafish.Zoological Lett. 2025 Jan 3;11(1):1. doi: 10.1186/s40851-024-00247-8. Zoological Lett. 2025. PMID: 39754210 Free PMC article.
-
The Nature and Origin of Synaptic Inputs to Vestibulospinal Neurons in the Larval Zebrafish.eNeuro. 2023 Jun 2;10(6):ENEURO.0090-23.2023. doi: 10.1523/ENEURO.0090-23.2023. Print 2023 Jun. eNeuro. 2023. PMID: 37268420 Free PMC article.
-
Cerebellar Purkinje cells control posture in larval zebrafish (Danio rerio).Elife. 2025 Apr 24;13:RP97614. doi: 10.7554/eLife.97614. Elife. 2025. PMID: 40272244 Free PMC article.
-
A connectomic resource for neural cataloguing and circuit dissection of the larval zebrafish brain.bioRxiv [Preprint]. 2025 Jun 15:2025.06.10.658982. doi: 10.1101/2025.06.10.658982. bioRxiv. 2025. PMID: 40661440 Free PMC article. Preprint.
References
-
- Morasso PBACMSV. Preflexes and internal models in biomimetic robot systems. Cogn. Process. 2005;6:25–36. doi: 10.1007/s10339-004-0039-6. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

