Clinical outcomes of patients diagnosed with cancer of unknown primary or malignancy of undefined primary origin who were referred to a regional cancer center
- PMID: 36899286
- PMCID: PMC10119062
- DOI: 10.1007/s10147-023-02316-y
Clinical outcomes of patients diagnosed with cancer of unknown primary or malignancy of undefined primary origin who were referred to a regional cancer center
Abstract
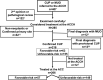
Background: A regional cancer hospital has been identified to be crucial in the management of malignancies of undefined primary origin (MUO) and cancer of unknown primary (CUP). This hospital primarily consists of oncologists with expertise in CUP, pathologists, and interventional radiologists. Early consultation or referral of MUO and CUP to a cancer hospital is deemed important.
Methods: This study retrospectively collected and analyzed the clinical, pathological, and outcome data of all patients (n = 407) referred to the Aichi Cancer Center Hospital (ACCH) in Japan over an 8-year period.
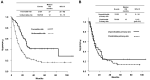
Results: In total, 30% of patients were referred for a second opinion. Among 285 patients, 13% had non-neoplastic disease or confirmed primary site and 76% had confirmed CUP (cCUP), with 29% of cCUP being identified as favorable risk. In 155 patients with unfavorable-risk CUP, 73% had primary sites predicted by immunohistochemistry (IHC) and distribution of metastatic sites, whereas 66% of them received site-specific therapies based on the predicted primary sites. The median overall survival (OS) was found to be poor in patients with MUO (1 month) and provisional CUP (6 months). In addition, the median OS of 206 patients with cCUP treated at the ACCH was 16 months (favorable risk, 27 months; unfavorable risk, 12 months). No significant difference was noted in OS between patients with non-predictable and predictable primary-sites (13 vs 12 months, p = 0.411).
Conclusion: The outcome of patients with unfavorable-risk CUP remains to be poor. Site-specific therapy based on IHC is not recommended for all patients with unfavorable-risk CUP.
Keywords: Cancer of unknown primary; Immunohistochemistry; Predicted primary site; Site-specific therapy; Unfavorable risk.
© 2023. The Author(s).
Conflict of interest statement
M Ando reports honoraria for lectures from Eisai Co., Ltd., Ono Pharmaceutical Co.,Ltd., Chugai Pharmaceutical Co., Ltd., Mundipharma Co., Ltd., and Taiho Pharmaceutical Co., Ltd. K Honda reports grants or contracts from Chugai Pharmaceutical Co., Ltd., and Taiho Pharmaceutical Co., Ltd. W Hosoda reports no conflicts of interest. Y Matsubara reports honoraria for lectures from Taiho Pharmaceutical Co., Ltd., Bristol Myers Squibb, Eli Lilly Japan K.K., Takeda Pharmaceutical Co. Ltd., and Merck Biopharma Co., Ltd. R Kumanishi reports no conflicts of interest. T Nakazawa reports honoraria for lectures from Eli Lilly Japan K.K. T Ogata reports honoraria for lectures from Ono Pharmaceutical Co.,Ltd., Taiho Pharmaceutical Co., Ltd., and Bristol Myers Squibb. A Nakata reports no conflicts of interest. H Kodama reports no conflicts of interest. T Masuishi reports grants or contracts from MSD K.K., Daiichi Sankyo Co.,Ltd., Ono Pharmaceutical Co.,Ltd., Novartis Pharma K.K., Amgen Inc., Syneos Health Clinical K.K., Boehringer Ingelheim, Pfizer Inc., CIMIC Shift Zero K.K., and Eli Lilly Japan K.K.; honoraria for lectures from Takeda Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co., Ltd., Merck Biopharma Co. Ltd., Taiho Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Eli Lilly Japan K.K., Yakult Honsha Co., Ltd., Sanofi K.K., Daiichi Sankyo Co., Ltd., Ono Pharmaceutical Co.,Ltd., and Bristol Myers Squibb. Y Narita reports grants or personal fees from Ono Pharmaceutical Co.,Ltd., and Bristol Myers Squibb; honoraria for lectures, presentations, or speaker bureaus from Yakult Honsha Co., Ltd., Taiho Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Daiichi Sankyo Co., Ltd., and Astra Zeneca. H Taniguchi reports grants or contracts from Takeda Pharmaceutical Co. Ltd., Daiichi Sankyo Co.,Ltd., and Ono Pharmaceutical Co.,Ltd.; honoraria for lectures, presentations, or speaker bureaus from Takeda Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Merck Biopharma Co. Ltd., Ono Pharmaceutical Co.,Ltd., and Chugai Pharmaceutical Co., Ltd. S Kadowaki reports grants or contracts from Taiho Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., MSD K.K., Chugai Pharmaceutical Co., Ltd., Nobelpharma Co., Ltd., Ono Pharmaceutical Co.,Ltd., Daiichi Sankyo Co., Ltd., and Janssen Pharma K.K.; honoraria for lectures from Eli Lilly Japan K.K., Taiho Pharmaceutical Co., Ltd., Ono Pharmaceutical Co.,Ltd., Bristol Myers Squibb, Chugai Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Merck Biopharma Co. Ltd., Daiichi Sankyo Co., Ltd., and Eisai Co., Ltd. K Muro reports study funding and support for Medical Writing/editing from Astellas Pharma Inc., and OPEN Health; grants or contracts from Solasia Pharma Inc., Merck Biopharma Co. Ltd., Daiichi Sankyo Co., Ltd., Parexel International Inc., Pfizer Inc., MSD K.K., Amgen Inc., Ono Pharmaceutical Co.,Ltd., Astellas Pharma Inc., Sanofi K.K., Taiho Pharmaceutical Co., Ltd., and Eisai Co., Ltd.; consulting fees from Astra Zeneca, Ono Pharmaceutical Co.,Ltd., and Amgen Inc.; honoraria for lectures, presentations, or speakers bureaus from Ono Pharmaceutical Co.,Ltd., Taiho Pharmaceutical Co., Ltd., Bristol Myers Squibb, and Eli Lilly Japan K.K.; participation on a Data Safety Monitoring Board or Advisory Board from Ono Pharmaceutical Co.,Ltd., MSD K.K., Astra Zeneca, Daiichi Sankyo Co., Ltd., and Solasia Pharma Inc.
Figures

References
-
- NICE. NICE clinical guideline. metastatic malignant disease of unknown primary origin. london: national institute for health and clinical excellence; 2010. https://www.nice.org.uk/Guidance/CG104. Accessed Dec 2021
MeSH terms
LinkOut - more resources
Full Text Sources

