Comparison of Fecal Microbiota Communities between Primiparous and Multiparous Cows during Non-Pregnancy and Pregnancy
- PMID: 36899725
- PMCID: PMC10000135
- DOI: 10.3390/ani13050869
Comparison of Fecal Microbiota Communities between Primiparous and Multiparous Cows during Non-Pregnancy and Pregnancy
Abstract
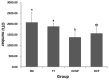
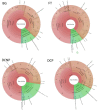
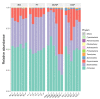
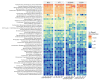
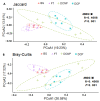
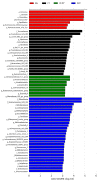
Imbalances in the gut microbiota composition may lead to several reproductive disorders and diseases during pregnancy. This study investigates the fecal microbiome composition between primiparous and multiparous cows during non-pregnancy and pregnancy to analyze the host-microbial balance at different stages. The fecal samples obtained from six cows before their first pregnancy (BG), six cows during their first pregnancy (FT), six open cows with more than three lactations (DCNP), and six pregnant cows with more than three lactations (DCP) were subjected to 16S rRNA sequencing, and a differential analysis of the fecal microbiota composition was performed. The three most abundant phyla in fecal microbiota were Firmicutes (48.68%), Bacteroidetes (34.45%), and Euryarchaeota (15.42%). There are 11 genera with more than 1.0% abundance at the genus level. Both alpha diversity and beta diversity showed significant differences among the four groups (p < 0.05). Further, primiparous women were associated with a profound alteration of the fecal microbiota. The most representative taxa included Rikenellaceae_RC9_gut_group, Prevotellaceae_UCG_003, Christensenellaceae_R_7_group, Ruminococcaceae UCG-005, Ruminococcaceae UCG-013, Ruminococcaceae UCG-014, Methanobrevibacter, and [Eubacterium] coprostanoligenes group, which were associated with energy metabolism and inflammation. The findings indicate that host-microbial interactions promote adaptation to pregnancy and will benefit the development of probiotics or fecal transplantation for treating dysbiosis and preventing disease development during pregnancy.
Keywords: 16S rRNA sequencing; cow; fecal microbiota; pregnancy; primiparous and multiparous.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Dynamic changes of the fecal bacterial community in dairy cows during early lactation.AMB Express. 2020 Sep 17;10(1):167. doi: 10.1186/s13568-020-01106-3. AMB Express. 2020. PMID: 32944794 Free PMC article.
-
Longitudinal Changes in Milk Microorganisms in the First Two Months of Lactation of Primiparous and Multiparous Cows.Animals (Basel). 2023 Jun 8;13(12):1923. doi: 10.3390/ani13121923. Animals (Basel). 2023. PMID: 37370433 Free PMC article.
-
Host Species Affects Bacterial Evenness, but Not Diversity: Comparison of Fecal Bacteria of Cows and Goats Offered the Same Diet.Animals (Basel). 2022 Aug 9;12(16):2011. doi: 10.3390/ani12162011. Animals (Basel). 2022. PMID: 36009603 Free PMC article.
-
The Response of Fecal Microbiota and Host Metabolome in Dairy Cows Following Rumen Fluid Transplantation.Front Microbiol. 2022 Jul 13;13:940158. doi: 10.3389/fmicb.2022.940158. eCollection 2022. Front Microbiol. 2022. PMID: 35923396 Free PMC article.
-
Shifting sows: longitudinal changes in the periparturient faecal microbiota of primiparous and multiparous sows.Animal. 2021 Mar;15(3):100135. doi: 10.1016/j.animal.2020.100135. Epub 2020 Dec 26. Animal. 2021. PMID: 33573959
Cited by
-
Changes in Gut Microbiota Associated with Parity in Large White Sows.Animals (Basel). 2023 Dec 28;14(1):112. doi: 10.3390/ani14010112. Animals (Basel). 2023. PMID: 38200843 Free PMC article.
-
Impact of a Saccharomyces cerevisiae fermentation product during an intestinal barrier challenge in lactating Holstein cows on ileal microbiota and markers of tissue structure and immunity.J Anim Sci. 2023 Jan 3;101:skad309. doi: 10.1093/jas/skad309. J Anim Sci. 2023. PMID: 37721866 Free PMC article.
-
Interactions between time on diet, antibiotic treatment, and liver abscess development on the fecal microbiome of beef cattle.Anim Microbiome. 2025 May 12;7(1):45. doi: 10.1186/s42523-025-00413-z. Anim Microbiome. 2025. PMID: 40355979 Free PMC article.
-
Physiological and Microbial Community Dynamics in Does During Mid-Gestation to Lactation and Their Impact on the Growth, Immune Function, and Microbiome Transmission of Offspring Kids.Animals (Basel). 2025 Mar 26;15(7):954. doi: 10.3390/ani15070954. Animals (Basel). 2025. PMID: 40218348 Free PMC article.
References
-
- Gradmark A., Pomeroy J., Renstrom F., Steiginga S., Persson M., Wright A., Bluck L., Domellof M., Kahn S.E., Mogren I., et al. Physical activity, sedentary behaviors, and estimated insulin sensitivity and secretion in pregnant and non-pregnant women. BMC Pregnancy Childb. 2011;11:44. doi: 10.1186/1471-2393-11-44. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

