Proliferating Astrocytes in Primary Culture Do Not Depend upon Mitochondrial Respiratory Complex I Activity or Oxidative Phosphorylation
- PMID: 36899819
- PMCID: PMC10001222
- DOI: 10.3390/cells12050683
Proliferating Astrocytes in Primary Culture Do Not Depend upon Mitochondrial Respiratory Complex I Activity or Oxidative Phosphorylation
Abstract
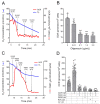
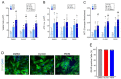
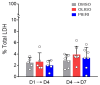
Understanding the role of astrocytes in the development of the nervous system and neurodegenerative disorders implies a necessary knowledge of the oxidative metabolism of proliferating astrocytes. The electron flux through mitochondrial respiratory complexes and oxidative phosphorylation may impact the growth and viability of these astrocytes. Here, we aimed at assessing to which extent mitochondrial oxidative metabolism is required for astrocyte survival and proliferation. Primary astrocytes from the neonatal mouse cortex were cultured in a physiologically relevant medium with the addition of piericidin A or oligomycin at concentrations that fully inhibit complex I-linked respiration and ATP synthase, respectively. The presence of these mitochondrial inhibitors for up to 6 days in a culture medium elicited only minor effects on astrocyte growth. Moreover, neither the morphology nor the proportion of glial fibrillary acidic protein-positive astrocytes in culture was affected by piericidin A or oligomycin. Metabolic characterization of the astrocytes showed a relevant glycolytic metabolism under basal conditions, despite functional oxidative phosphorylation and large spare respiratory capacity. Our data suggest that astrocytes in primary culture can sustainably proliferate when their energy metabolism relies only on aerobic glycolysis since their growth and survival do not require electron flux through respiratory complex I or oxidative phosphorylation.
Keywords: OXPHOS; astrocytes; bioenergetic; mitochondria; oligomycin; piericidin A.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

