Intrahippocampal Inoculation of Aβ1-42 Peptide in Rat as a Model of Alzheimer's Disease Identified MicroRNA-146a-5p as Blood Marker with Anti-Inflammatory Function in Astrocyte Cells
- PMID: 36899831
- PMCID: PMC10000752
- DOI: 10.3390/cells12050694
Intrahippocampal Inoculation of Aβ1-42 Peptide in Rat as a Model of Alzheimer's Disease Identified MicroRNA-146a-5p as Blood Marker with Anti-Inflammatory Function in Astrocyte Cells
Abstract
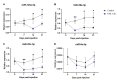
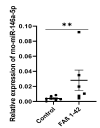
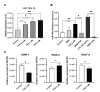
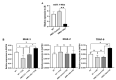
Circulating microRNAs (miRNAs) have aroused a lot of interest as reliable blood diagnostic biomarkers of Alzheimer's disease (AD). Here, we investigated the panel of expressed blood miRNAs in response to aggregated Aβ1-42 peptides infused in the hippocampus of adult rats to mimic events of the early onset of non-familial AD disorder. Aβ1-42 peptides in the hippocampus led to cognitive impairments associated with an astrogliosis and downregulation of circulating miRNA-146a-5p, -29a-3p, -29c-3p, -125b-5p, and-191-5p. We established the kinetics of expression of selected miRNAs and found differences with those detected in the APPswe/PS1dE9 transgenic mouse model. Of note, miRNA-146a-5p was exclusively dysregulated in the Aβ-induced AD model. The treatment of primary astrocytes with Aβ1-42 peptides led to miRNA-146a-5p upregulation though the activation of the NF-κB signaling pathway, which in turn downregulated IRAK-1 but not TRAF-6 expression. As a consequence, no induction of IL-1β, IL-6, or TNF-α was detected. Astrocytes treated with a miRNA-146-5p inhibitor rescued IRAK-1 and changed TRAF-6 steady-state levels that correlated with the induction of IL-6, IL-1β, and CXCL1 production, indicating that miRNA-146a-5p operates anti-inflammatory functions through a NF-κB pathway negative feedback loop. Overall, we report a panel of circulating miRNAs that correlated with Aβ1-42 peptides' presence in the hippocampus and provide mechanistic insights into miRNA-146a-5p biological function in the development of the early stage of sporadic AD.
Keywords: Alzheimer’s disease; Aβ1–42 peptide; biomarkers; diagnosis; miRNA-146a-5p; microRNAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

