Role of RUNX3 in Restriction Point Regulation
- PMID: 36899846
- PMCID: PMC10000377
- DOI: 10.3390/cells12050708
Role of RUNX3 in Restriction Point Regulation
Abstract
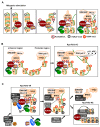
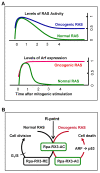
A cell cycle is a series of events that takes place in a cell as it grows and divides. At the G1 phase of cell cycle, cells monitor their cumulative exposure to specific signals and make the critical decision to pass through the restriction (R)-point. The R-point decision-making machinery is fundamental to normal differentiation, apoptosis, and G1-S transition. Deregulation of this machinery is markedly associated with tumorigenesis. Therefore, identification of the molecular mechanisms that govern the R-point decision is one of the fundamental issues in tumor biology. RUNX3 is one of the genes frequently inactivated in tumors by epigenetic alterations. In particular, RUNX3 is downregulated in most K-RAS-activated human and mouse lung adenocarcinomas (ADCs). Targeted inactivation of Runx3 in the mouse lung induces adenomas (ADs), and markedly shortens the latency of ADC formation induced by oncogenic K-Ras. RUNX3 participates in the transient formation of R-point-associated activator (RPA-RX3-AC) complexes, which measure the duration of RAS signals and thereby protect cells against oncogenic RAS. This review focuses on the molecular mechanism by which the R-point participates in oncogenic surveillance.
Keywords: K-RAS; R-point; RUNX3; cell cycle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Weinberg R.A. The Biology of Cancer. Garland Science; New York, NY, USA: 2007. pp. 275–329. Chapter 8. pRB and Control of the Cell Cycle Clock.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

