Upregulation of TLR4-Dependent ATP Production Is Critical for Glaesserella parasuis LPS-Mediated Inflammation
- PMID: 36899887
- PMCID: PMC10001010
- DOI: 10.3390/cells12050751
Upregulation of TLR4-Dependent ATP Production Is Critical for Glaesserella parasuis LPS-Mediated Inflammation
Abstract
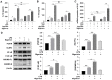
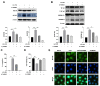
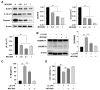
Glaesserella parasuis (G. parasuis), an important pathogenic bacterium, cause Glässer's disease, and has resulted in tremendous economic losses to the global swine industry. G. parasuis infection causes typical acute systemic inflammation. However, the molecular details of how the host modulates the acute inflammatory response induced by G. parasuis are largely unknown. In this study, we found that G. parasuis LZ and LPS both enhanced the mortality of PAM cells, and at the same time, the level of ATP was enhanced. LPS treatment significantly increased the expressions of IL-1β, P2X7R, NLRP3, NF-κB, p-NF-κB, and GSDMD, leading to pyroptosis. Furthermore, these proteins' expression was enhanced following extracellular ATP further stimulation. When reduced the production of P2X7R, NF-κB-NLRP3-GSDMS inflammasome signaling pathway was inhibited, and the mortality of cells was reduced. MCC950 treatment repressed the formation of inflammasome and reduced mortality. Further exploration found that the knockdown of TLR4 significantly reduced ATP content and cell mortality, and inhibited the expression of p-NF-κB and NLRP3. These findings suggested upregulation of TLR4-dependent ATP production is critical for G. parasuis LPS-mediated inflammation, provided new insights into the molecular pathways underlying the inflammatory response induced by G. parasuis, and offered a fresh perspective on therapeutic strategies.
Keywords: ATP; G. parasuis LPS; P2X7R; TLR4; acute inflammatory response; inflammation; pharmacological target.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Baicalin suppresses NLRP3 inflammasome and nuclear factor-kappa B (NF-κB) signaling during Haemophilus parasuis infection.Vet Res. 2016 Aug 8;47(1):80. doi: 10.1186/s13567-016-0359-4. Vet Res. 2016. PMID: 27502767 Free PMC article.
-
Glaesserella parasuis induces inflammatory response in 3D4/21 cells through activation of NLRP3 inflammasome signaling pathway via ROS.Vet Microbiol. 2021 May;256:109057. doi: 10.1016/j.vetmic.2021.109057. Epub 2021 Mar 26. Vet Microbiol. 2021. PMID: 33799227
-
Glaesserella parasuis serotype 5 induces pyroptosis via the RIG-I/MAVS/NLRP3 pathway in swine tracheal epithelial cells.Vet Microbiol. 2024 Jul;294:110127. doi: 10.1016/j.vetmic.2024.110127. Epub 2024 May 22. Vet Microbiol. 2024. PMID: 38797057
-
CD200Fc reduces LPS-induced IL-1β activation in human cervical cancer cells by modulating TLR4-NF-κB and NLRP3 inflammasome pathway.Oncotarget. 2017 May 16;8(20):33214-33224. doi: 10.18632/oncotarget.16596. Oncotarget. 2017. PMID: 28402258 Free PMC article.
-
Isoliquiritigenin alleviates P. gingivalis-LPS/ATP-induced pyroptosis by inhibiting NF-κB/ NLRP3/GSDMD signals in human gingival fibroblasts.Int Immunopharmacol. 2021 Dec;101(Pt B):108338. doi: 10.1016/j.intimp.2021.108338. Epub 2021 Nov 15. Int Immunopharmacol. 2021. PMID: 34794890
Cited by
-
Baicalin Alleviates ADAM17/EGFR Axis-Induced Peritonitis in Weaned Piglets Infected by Glaesserella parasuis.Animals (Basel). 2025 Aug 21;15(16):2457. doi: 10.3390/ani15162457. Animals (Basel). 2025. PMID: 40867785 Free PMC article.
-
Baicalin and probenecid protect against Glaesserella parasuis challenge in a piglet model.Vet Res. 2024 Jul 29;55(1):96. doi: 10.1186/s13567-024-01352-4. Vet Res. 2024. PMID: 39075542 Free PMC article.
-
Baicalin attenuates PD-1/PD-L1 axis-induced immunosuppression in piglets challenged with Glaesserella parasuis by inhibiting the PI3K/Akt/mTOR and RAS/MEK/ERK signalling pathways.Vet Res. 2024 Jul 29;55(1):95. doi: 10.1186/s13567-024-01355-1. Vet Res. 2024. PMID: 39075562 Free PMC article.
-
Baicalin Relieves Glaesserella parasuis-Triggered Immunosuppression Through Polarization via MIF/CD74 Signaling Pathway in Piglets.Biomolecules. 2025 Apr 29;15(5):640. doi: 10.3390/biom15050640. Biomolecules. 2025. PMID: 40427533 Free PMC article.
-
Gut microbiota in health and disease: advances and future prospects.MedComm (2020). 2024 Nov 20;5(12):e70012. doi: 10.1002/mco2.70012. eCollection 2024 Dec. MedComm (2020). 2024. PMID: 39568773 Free PMC article. Review.
References
-
- Matiaskova K., Kavanova L., Kulich P., Gebauer J., Nedbalcova K., Kudlackova H., Tesarik R., Faldyna M. The Role of Antibodies Against the Crude Capsular Extract in the Immune Response of Porcine Alveolar Macrophages to in Vitro Infection of Various Serovars of Glaesserella (Haemophilus) parasuis. Front. Immunol. 2021;12:635097. doi: 10.3389/fimmu.2021.635097. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

