Polyunsaturated Fatty Acids Drive Lipid Peroxidation during Ferroptosis
- PMID: 36899940
- PMCID: PMC10001165
- DOI: 10.3390/cells12050804
Polyunsaturated Fatty Acids Drive Lipid Peroxidation during Ferroptosis
Abstract
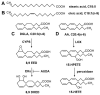
Ferroptosis is a form of regulated cell death that is intricately linked to cellular metabolism. In the forefront of research on ferroptosis, the peroxidation of polyunsaturated fatty acids has emerged as a key driver of oxidative damage to cellular membranes leading to cell death. Here, we review the involvement of polyunsaturated fatty acids (PUFAs), monounsaturated fatty acids (MUFAs), lipid remodeling enzymes and lipid peroxidation in ferroptosis, highlighting studies revealing how using the multicellular model organism Caenorhabditis elegans contributes to the understanding of the roles of specific lipids and lipid mediators in ferroptosis.
Keywords: AA; Caenorhabditis elegans; DGLA; MUFA; PUFA; ferroptosis; lipid peroxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Dixon S.J., Stockwell B.R. The Hallmarks of Ferroptosis. Annu. Rev. Cancer Biol. 2019;3:35–54. doi: 10.1146/annurev-cancerbio-030518-055844. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

