Diagnostic Performance of Selected MRI-Derived Radiomics Able to Discriminate Progression-Free and Overall Survival in Patients with Midline Glioma and the H3F3AK27M Mutation
- PMID: 36899993
- PMCID: PMC10001394
- DOI: 10.3390/diagnostics13050849
Diagnostic Performance of Selected MRI-Derived Radiomics Able to Discriminate Progression-Free and Overall Survival in Patients with Midline Glioma and the H3F3AK27M Mutation
Abstract
Background: Radiomics refers to a recent area of knowledge that studies features extracted from different imaging techniques and subsequently transformed into high-dimensional data that can be associated with biological events. Diffuse midline gliomas (DMG) are one of the most devastating types of cancer, with a median survival of approximately 11 months after diagnosis and 4-5 months after radiological and clinical progression.
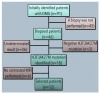
Methods: A retrospective study. From a database of 91 patients with DMG, only 12 had the H3.3K27M mutation and brain MRI DICOM files available. Radiomic features were extracted from MRI T1 and T2 sequences using LIFEx software. Statistical analysis included normal distribution tests and the Mann-Whitney U test, ROC analysis, and calculation of cut-off values.
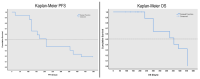
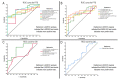
Results: A total of 5760 radiomic values were included in the analyses. AUROC demonstrated 13 radiomics with statistical significance for progression-free survival (PFS) and overall survival (OS). Diagnostic performance tests showed nine radiomics with specificity for PFS above 90% and one with a sensitivity of 97.2%. For OS, 3 out of 4 radiomics demonstrated between 80 and 90% sensitivity.
Conclusions: Several radiomic features demonstrated statistical significance and have the potential to further aid DMG diagnostic assessment non-invasively. The most significant radiomics were first- and second-order features with GLCM texture profile, GLZLM_GLNU, and NGLDM_Contrast.
Keywords: MRI; brain tumour; midline glioma; paediatric; prognosis; radiomic.
Conflict of interest statement
The authors declare that there are no conflict of interest regarding the publication of this article.
Figures


Similar articles
-
Identification of Radiomic Signatures in Brain MRI Sequences T1 and T2 That Differentiate Tumor Regions of Midline Gliomas with H3.3K27M Mutation.Diagnostics (Basel). 2023 Aug 14;13(16):2669. doi: 10.3390/diagnostics13162669. Diagnostics (Basel). 2023. PMID: 37627927 Free PMC article.
-
Radiomic Features Based on MRI Predict Progression-Free Survival in Pediatric Diffuse Midline Glioma/Diffuse Intrinsic Pontine Glioma.Can Assoc Radiol J. 2023 Feb;74(1):119-126. doi: 10.1177/08465371221109921. Epub 2022 Jun 29. Can Assoc Radiol J. 2023. PMID: 35768942
-
Exploring MRI Characteristics of Brain Diffuse Midline Gliomas With the H3 K27M Mutation Using Radiomics.Front Oncol. 2021 May 24;11:646267. doi: 10.3389/fonc.2021.646267. eCollection 2021. Front Oncol. 2021. PMID: 34109112 Free PMC article.
-
Development and validation of a machine learning algorithm for predicting diffuse midline glioma, H3 K27-altered, H3 K27 wild-type high-grade glioma, and primary CNS lymphoma of the brain midline in adults.J Neurosurg. 2022 Dec 23;139(2):393-401. doi: 10.3171/2022.11.JNS221544. Print 2023 Aug 1. J Neurosurg. 2022. PMID: 36681946
-
Noninvasive Determination of IDH and 1p19q Status of Lower-grade Gliomas Using MRI Radiomics: A Systematic Review.AJNR Am J Neuroradiol. 2021 Jan;42(1):94-101. doi: 10.3174/ajnr.A6875. Epub 2020 Nov 26. AJNR Am J Neuroradiol. 2021. PMID: 33243896 Free PMC article.
Cited by
-
Radiomics and artificial intelligence applications in pediatric brain tumors.World J Pediatr. 2024 Aug;20(8):747-763. doi: 10.1007/s12519-024-00823-0. Epub 2024 Jun 27. World J Pediatr. 2024. PMID: 38935233 Free PMC article. Review.
-
The value of MRI-based radiomics for evaluating early parotid gland injury in primary Sjögren's syndrome.Clin Rheumatol. 2024 May;43(5):1675-1682. doi: 10.1007/s10067-024-06935-2. Epub 2024 Mar 27. Clin Rheumatol. 2024. PMID: 38538907
-
Systematic review and epistemic meta-analysis to advance binomial AI-radiomics integration for predicting high-grade glioma progression and enhancing patient management.Sci Rep. 2025 May 8;15(1):16113. doi: 10.1038/s41598-025-98058-0. Sci Rep. 2025. PMID: 40341184 Free PMC article.
-
Identification of Radiomic Signatures in Brain MRI Sequences T1 and T2 That Differentiate Tumor Regions of Midline Gliomas with H3.3K27M Mutation.Diagnostics (Basel). 2023 Aug 14;13(16):2669. doi: 10.3390/diagnostics13162669. Diagnostics (Basel). 2023. PMID: 37627927 Free PMC article.
-
MRI radiomics based on machine learning in high-grade gliomas as a promising tool for prediction of CD44 expression and overall survival.Sci Rep. 2025 Mar 3;15(1):7433. doi: 10.1038/s41598-025-90128-7. Sci Rep. 2025. PMID: 40032983 Free PMC article.
References
-
- Gianno F., Giovannoni I., Cafferata B., Diomedi-Camassei F., Minasi S., Barresi S., Buttarelli F.R., Alesi V., Cardoni A., Antonelli M., et al. Paediatric-type diffuse high-grade gliomas in the 5th CNS WHO Classification. Pathologica. 2022;114:422–435. doi: 10.32074/1591-951X-830. - DOI - PMC - PubMed
-
- Mackay A., Burford A., Carvalho D., Izquierdo E., Fazal-Salom J., Taylor K.R., Bjerke L., Clarke M., Vinci M., Nandhabalan M., et al. Integrated Molecular Meta-Analysis of 1000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell. 2017;32:520–537.e5. doi: 10.1016/j.ccell.2017.08.017. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

