Impact of Novel Treatments in Patients with Melanoma Brain Metastasis: Real-World Data
- PMID: 36900253
- PMCID: PMC10000692
- DOI: 10.3390/cancers15051461
Impact of Novel Treatments in Patients with Melanoma Brain Metastasis: Real-World Data
Abstract
Background: Melanoma brain metastasis (MBM) is associated with poor outcome, but targeted therapies (TTs) and immune checkpoint inhibitors (ICIs) have revolutionized treatment over the past decade. We assessed the impact of these treatments in a real-world setting.
Methods: A single-center cohort study was performed at a large, tertiary referral center for melanoma (Erasmus MC, Rotterdam, the Netherlands). Overall survival (OS) was assessed before and after 2015, after which TTs and ICIs were increasingly prescribed.
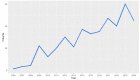
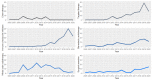
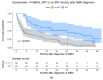
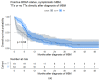
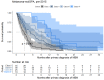
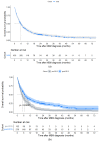
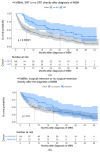
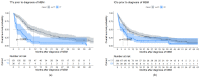
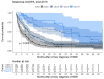
Results: There were 430 patients with MBM included; 152 pre-2015 and 278 post-2015. Median OS improved from 4.4 to 6.9 months (HR 0.67, p < 0.001) after 2015. TTs and ICIs prior to MBM diagnosis were associated with poorer median OS as compared to no prior systemic treatment (TTs: 2.0 vs. 10.9 and ICIs: 4.2 vs. 7.9 months, p < 0.001). ICIs directly after MBM diagnosis were associated with improved median OS as compared to no direct ICIs (21.5 vs. 4.2 months, p < 0.001). Stereotactic radiotherapy (SRT; HR 0.49, p = 0.013) and ICIs (HR 0.32, p < 0.001) were independently associated with improved OS.
Conclusion: After 2015, OS significantly improved for patients with MBM, especially with SRT and ICIs. Demonstrating a large survival benefit, ICIs should be considered first after MBM diagnosis, if clinically feasible.
Keywords: BRAF/MEK inhibitors; brain neoplasms/metastasis; immune checkpoint inhibitors; immunotherapy; melanoma; molecular targeted therapy; radiotherapy; survival.
Conflict of interest statement
A.A.M.v.d.V.: consultancy boards (fees paid to the institution) for BMS, MSD, Merck, Sanofi, Pierre Fabre, Roche, Novartis, Pfizer, Eisai, Ipsen. These funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. All other authors declare no conflict of interest.
Figures




References
-
- Cagney D.N., Martin A.M., Catalano P.J., Redig A.J., Lin N.U., Lee E.Q., Wen P.Y., Dunn I.F., Bi W.L., Weiss S.E., et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro. Oncol. 2017;19:1511–1521. doi: 10.1093/neuonc/nox077. - DOI - PMC - PubMed
-
- Cohen J.V., Tawbi H., Margolin K.A., Amravadi R., Bosenberg M., Brastianos P.K., Chiang V.L., de Groot J., Glitza I.C., Herlyn M., et al. Melanoma central nervous system metastases: Current approaches, challenges, and opportunities. Pigment. Cell Melanoma Res. 2016;29:627–642. doi: 10.1111/pcmr.12538. - DOI - PMC - PubMed
-
- Chang E.L., Wefel J.S., Hess K.R., Allen P.K., Lang F.F., Kornguth D.G., Arbuckle R.B., Swint J.M., Shiu A.S., Maor M.H., et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

