Technical Implications for Surgical Resection in Locally Advanced Pancreatic Cancer
- PMID: 36900300
- PMCID: PMC10000506
- DOI: 10.3390/cancers15051509
Technical Implications for Surgical Resection in Locally Advanced Pancreatic Cancer
Abstract
Pancreatic ductal adenocarcinoma remains a global health challenge and is predicted to soon become the second leading cause of cancer death in developed countries. Currently, surgical resection in combination with systemic chemotherapy offers the only chance of cure or long-term survival. However, only 20% of cases are diagnosed with anatomically resectable disease. Neoadjuvant treatment followed by highly complex surgical procedures has been studied over the last decade with promising short- and long-term results in patients with locally advanced pancreatic ductal adenocarcinoma (LAPC). In recent years, a wide variety of complex surgical techniques that involve extended pancreatectomies, including portomesenteric venous resection, arterial resection, or multi-organ resection, have emerged to optimize local control of the disease and improve postoperative outcomes. Although there are multiple surgical techniques described in the literature to improve outcomes in LAPC, the comprehensive view of these strategies remains underdeveloped. We aim to describe the preoperative surgical planning as well different surgical resections strategies in LAPC after neoadjuvant treatment in an integrated way for selected patients with no other potentially curative option other than surgery.
Keywords: arterial resection; extended pancreatectomies; locally advanced pancreatic ductal adenocarcinoma; neoadjuvant treatment; portomesenteric venous resection.
Conflict of interest statement
None of the authors of this manuscript have any direct or indirect commercial financial incentive associated with the publication of this paper.
Figures
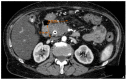

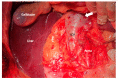
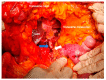
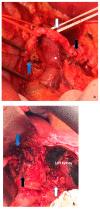




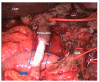
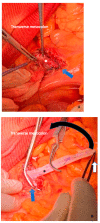
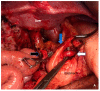

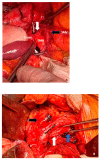
References
-
- Klaiber U., Schnaidt E.S., Hinz U., Gaida M.M., Heger U., Hank T., Strobel O., Neoptolemos J.P., Mihaljevic A.L., Büchler M.W., et al. Prognostic Factors of Survival After Neoadjuvant Treatment and Resection for Initially Unresectable Pancreatic Cancer. Ann. Surg. 2021;273:154–162. doi: 10.1097/SLA.0000000000003270. - DOI - PubMed
-
- Amin M.B., Greene F.L., Edge S.B., Compton C.C., Gershenwald J.E., Brookland R.K., Meyer L., Gress D.M., Byrd D.R., Winchester D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017;67:93–99. doi: 10.3322/caac.21388. - DOI - PubMed
-
- Bockhorn M., Uzunoglu F.G., Adham M., Imrie C., Milicevic M., Sandberg A.A., Asbun H.J., Bassi C., Büchler M., Charnley R.M., et al. International Study Group of Pancreatic Surgery. Borderline resectable pancreatic cancer: A consensus statement by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2014;155:977–988. doi: 10.1016/j.surg.2014.02.001. - DOI - PubMed
-
- Kirkegård J., Aahlin E.K., Al-Saiddi M., Bratlie S.O., Coolsen M., de Haas R.J., den Dulk M., Fristrup C., Harrison E.M., Mortensen M.B., et al. Multicentre study of multidisciplinary team assessment of pancreatic cancer resectability and treatment allocation. Br. J. Surg. 2019;106:756–764. doi: 10.1002/bjs.11093. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

