B Cells in Breast Cancer Pathology
- PMID: 36900307
- PMCID: PMC10000926
- DOI: 10.3390/cancers15051517
B Cells in Breast Cancer Pathology
Abstract
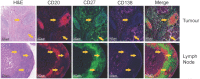
B cells have recently become a focus in breast cancer pathology due to their influence on tumour regression, prognosis, and response to treatment, besides their contribution to antigen presentation, immunoglobulin production, and regulation of adaptive responses. As our understanding of diverse B cell subsets in eliciting both pro- and anti-inflammatory responses in breast cancer patients increases, it has become pertinent to address the molecular and clinical relevance of these immune cell populations within the tumour microenvironment (TME). At the primary tumour site, B cells are either found spatially dispersed or aggregated in so-called tertiary lymphoid structures (TLS). In axillary lymph nodes (LNs), B cell populations, amongst a plethora of activities, undergo germinal centre reactions to ensure humoral immunity. With the recent approval for the addition of immunotherapeutic drugs as a treatment option in the early and metastatic settings for triple-negative breast cancer (TNBC) patients, B cell populations or TLS may resemble valuable biomarkers for immunotherapy responses in certain breast cancer subgroups. New technologies such as spatially defined sequencing techniques, multiplex imaging, and digital technologies have further deciphered the diversity of B cells and the morphological structures in which they appear in the tumour and LNs. Thus, in this review, we comprehensively summarise the current knowledge of B cells in breast cancer. In addition, we provide a user-friendly single-cell RNA-sequencing platform, called "B singLe cEll rna-Seq browSer" (BLESS) platform, with a focus on the B cells in breast cancer patients to interrogate the latest publicly available single-cell RNA-sequencing data collected from diverse breast cancer studies. Finally, we explore their clinical relevance as biomarkers or molecular targets for future interventions.
Keywords: B cells; breast cancer; germinal centres; lymph nodes; tertiary lymphoid structures; tumour-infiltrating lymphocytes.
Conflict of interest statement
The authors declare the following financial interests/personal relationships, which may be considered potential competing interests: A.Q is an AstraZeneca employee.
Figures

References
-
- Loi S., Drubay D., Adams S., Pruneri G., Francis P.A., Lacroix-Triki M., Joensuu H., Dieci M.V., Badve S., Demaria S., et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019;37:559–569. doi: 10.1200/JCO.18.01010. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

