Impact of the 21-Gene Assay in Patients with High-Clinical Risk ER-Positive and HER2-Negative Early Breast Cancer: Results of the KARMA Dx Study
- PMID: 36900321
- PMCID: PMC10001004
- DOI: 10.3390/cancers15051529
Impact of the 21-Gene Assay in Patients with High-Clinical Risk ER-Positive and HER2-Negative Early Breast Cancer: Results of the KARMA Dx Study
Abstract
Background: The 21-gene Oncotype DX Breast Recurrence Score® assay is prognostic and predictive of chemotherapy benefit for patients with estrogen receptor-positive, HER2- early breast cancer (EBC). The KARMA Dx study evaluated the impact of the Recurrence Score® results (RS) on the treatment decision for patients with EBC and high-risk clinicopathological characteristics for whom chemotherapy (CT) was considered.
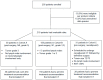
Methods: Eligible patients with EBC were candidates for the study if CT was considered standard recommendation by local guidelines. Three high-risk EBC cohorts were predefined: (A) pT1-2, pN0/N1mi, and grade 3; (B) pT1-2, pN1, and grades 1-2; and (C) neoadjuvant cT2-3, cN0, and Ki67 ≤ 30%. Treatment recommendations before and after 21-gene testing were registered, as well as treatment received and physicians' confidence levels in their final recommendations.
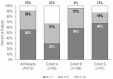
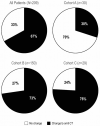
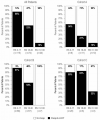
Results: A total of 219 consecutive patients were included from eight Spanish centers: 30 in cohort A, 158 in cohort B, and 31 in cohort C. Ten patients were excluded from the final analysis as CT was not initially recommended. After 21-gene testing, treatment decisions changed from CT + endocrine therapy (ET) to ET alone for 67% of the whole group. In total, 30% (95% confidence interval [CI] 15% to 49%), 73% (95% CI 65% to 80%), and 76% (95% CI 56% to 90%) of patients ultimately received ET alone in cohorts A, B, and C, respectively. Physicians' confidence in their final recommendations increased in 34% of cases.
Conclusions: Use of the 21-gene test resulted in an overall 67% reduction in CT recommendation in patients considered candidates for CT. Our findings indicate the substantial potential of the 21-gene test to guide CT recommendations in patients with EBC considered to be at high risk of recurrence based on clinicopathological parameters, regardless of nodal status or treatment setting.
Keywords: Oncotype DX Breast Recurrence Score®; Recurrence Score® result; adjuvant; breast cancer; chemotherapy; clinical utility.
Conflict of interest statement
Llombart-Cussac reported playing a leadership role at Eisai, Celgene, Lilly, Pfizer, Roche, Novartis, and MSD; intellectual property for MedSIR; a consulting role for Lilly, Roche, Pfizer, Novartis, Pierre-Fabre, ExactSciences, Seagen, and GSK; to be part of the speaker bureau for Lilly, AstraZeneca, and MSD; to receive research funding from Pfizer, Roche, Foundation Medicine, ExactSciences, Pierre-Fabre, and Agendia, and travel compensation from Roche, Lilly, Novartis, Pfizer, and AstraZeneca.
Figures

References
-
- Sparano J.A., Gray R.J., Makower D.F., Pritchard K.I., Albain K.S., Hayes D.F., Geyer C.E., Jr., Dees E.C., Goetz M.P., Olson J.A., Jr., et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N. Engl. J. Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. - DOI - PMC - PubMed
-
- Sparano J.A., Gray R.J., Makower D.F., Albain K.S., Saphner T.J., Badve S.S., Wagner L.I., Kaklamani V.G., Keane M.M., Gomez H.L., et al. Clinical outcomes in early breast cancer with a high 21-gene Recurrence Score of 26 to 100 assigned to adjuvant chemotherapy plus endocrine therapy: A secondary analysis of the TAILORx randomized clinical trial. JAMA Oncol. 2020;6:367–374. doi: 10.1001/jamaoncol.2019.4794. - DOI - PMC - PubMed
-
- Hortobagyi G.N., Connolly J.L., D’Orsi C.J., Edge S.B., Mitendorf E.A., Rugo H.S., Solin L.J., Weaver D.L., Winchester D.J., Giuliano A. Breast. In: Amin M.B., Edge S., Greene F., Byrd D.R., Brookland R.K., Washington M.K., Gershenwald J.E., Compton C.C., Hess K.R., Sullivan D.C., et al., editors. AJCC Cancer Staging Manual. 8th ed. Springer Publishing; New York, NY, USA: 2017. pp. 589–628.
-
- Andre F., Ismaila N., Henry N.L., Somerfield M.R., Bast R.C., Barlow W., Collyar D.E., Hammond M.E., Kuderer N.M., Liu M.C., et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO Clinical Practice Guideline update-Integration of results from TAILORx. J. Clin. Oncol. 2019;37:1956–1964. doi: 10.1200/JCO.19.00945. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

