Exploring Novel Therapeutic Opportunities for Glioblastoma Using Patient-Derived Cell Cultures
- PMID: 36900355
- PMCID: PMC10000883
- DOI: 10.3390/cancers15051562
Exploring Novel Therapeutic Opportunities for Glioblastoma Using Patient-Derived Cell Cultures
Abstract
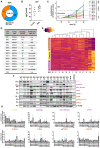
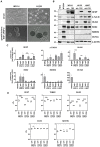
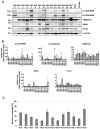
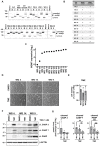
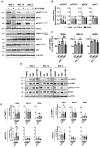
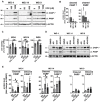
Glioblastomas (GBM) are the most common, primary brain tumors in adults. Despite advances in neurosurgery and radio- and chemotherapy, the median survival of GBM patients is 15 months. Recent large-scale genomic, transcriptomic and epigenetic analyses have shown the cellular and molecular heterogeneity of GBMs, which hampers the outcomes of standard therapies. We have established 13 GBM-derived cell cultures from fresh tumor specimens and characterized them molecularly using RNA-seq, immunoblotting and immunocytochemistry. Evaluation of proneural (OLIG2, IDH1R132H, TP53 and PDGFRα), classical (EGFR) and mesenchymal markers (CHI3L1/YKL40, CD44 and phospho-STAT3), and the expression of pluripotency (SOX2, OLIG2, NESTIN) and differentiation (GFAP, MAP2, β-Tubulin III) markers revealed the striking intertumor heterogeneity of primary GBM cell cultures. Upregulated expression of VIMENTIN, N-CADHERIN and CD44 at the mRNA/protein levels suggested increased epithelial-to-mesenchymal transition (EMT) in most studied cell cultures. The effects of temozolomide (TMZ) or doxorubicin (DOX) were tested in three GBM-derived cell cultures with different methylation status of the MGMT promoter. Amongst TMZ- or DOX-treated cultures, the strongest accumulation of the apoptotic markers caspase 7 and PARP were found in WG4 cells with methylated MGMT, suggesting that its methylation status predicts vulnerability to both drugs. As many GBM-derived cells showed high EGFR levels, we tested the effects of AG1478, an EGFR inhibitor, on downstream signaling pathways. AG1478 caused decreased levels of phospho-STAT3, and thus inhibition of active STAT3 augmented antitumor effects of DOX and TMZ in cells with methylated and intermediate status of MGMT. Altogether, our findings show that GBM-derived cell cultures mimic the considerable tumor heterogeneity, and that identifying patient-specific signaling vulnerabilities can assist in overcoming therapy resistance, by providing personalized combinatorial treatment recommendations.
Keywords: EGFR inhibitor (AG1478); EMT; MGMT; STAT3; cancer stem cells; doxorubicin; glioblastoma; temozolomide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kokkinakis D.M., Hoffman R.M., Frenkel E.P., Wick J.B., Han Q., Xu M., Tan Y., Schold S.C. Synergy between Methionine Stress and Chemotherapy in the Treatment of Brain Tumor Xenografts in Athymic Mice. Cancer Res. 2001;61:4017–4023. - PubMed
-
- Rivera A.L., Pelloski C.E., Gilbert M.R., Colman H., De La Cruz C., Sulman E.P., Bekele B.N., Aldape K.D. MGMT Promoter Methylation Is Predictive of Response to Radiotherapy and Prognostic in the Absence of Adjuvant Alkylating Chemotherapy for Glioblastoma. Neuro Oncol. 2010;12:116–121. doi: 10.1093/neuonc/nop020. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

