Atmospheric Deposition around the Industrial Areas of Milazzo and Priolo Gargallo (Sicily-Italy)-Part A: Major Ions
- PMID: 36900908
- PMCID: PMC10002272
- DOI: 10.3390/ijerph20053898
Atmospheric Deposition around the Industrial Areas of Milazzo and Priolo Gargallo (Sicily-Italy)-Part A: Major Ions
Abstract
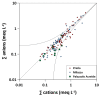
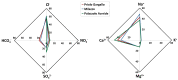
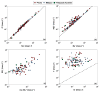
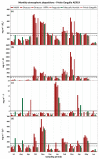
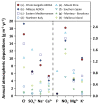
The chemical composition of rainwater was studied in two highly-industrialised areas in Sicily (southern Italy), between June 2018 and July 2019. The study areas were characterised by large oil refining plants and other industrial hubs whose processes contribute to the release of large amounts of gaseous species that can affect the chemical composition of atmospheric deposition As in most of the Mediterranean area, rainwater acidity (ranging in the study area between 3.9 and 8.3) was buffered by the dissolution of abundant geogenic carbonate aerosol. In particular, calcium and magnesium cations showed the highest pH-neutralizing factor, with ~92% of the acidity brought by SO42- and NO3- neutralized by alkaline dust. The lowest pH values were observed in samples collected after abundant rain periods, characterised by a less significant dry deposition of alkaline materials. Electrical Conductivity (ranging between 7 µS cm-1 and 396 µS cm-1) was inversely correlated with the amount of rainfall measured in the two areas. Concentrations of major ionic species followed the sequence Cl- > Na+ > SO42- ≃ HCO3- > ≃ Ca2+ > NO3- > Mg2+ > K+ > F-. High loads of Na+ and Cl- (with a calculated R2 = 0.99) reflected proximity to the sea. Calcium, potassium, and non-sea-salt magnesium had a prevalent crustal origin. Non-sea salt sulphate, nitrate, and fluoride can be attributed mainly to anthropogenic sources. Mt. Etna, during eruptive periods, may be also considered, on a regional scale, a significant source for fluoride, non-sea salt sulphate, and even chloride.
Keywords: acidity neutralization; anthropogenic source; atmospheric deposition; major ions; marine source.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- D’Alessandro W., Katsanou K., Lambrakis N., Bellomo S., Brusca L., Liotta M. Chemical and isotopic characterisation of bulk deposition in the Louros basin (Epirus, Greece) Atmos. Res. 2013;132–133:399–410. doi: 10.1016/j.atmosres.2013.07.007. - DOI
-
- Galy-Lacaux C., Laouali D., Descroix L., Gobron N., Liousse C. Long term precipitation chemistry and wet deposition in a remote dry savanna site in Africa (Niger) Atmos. Chem. Phys. 2009;9:1579–1595. doi: 10.5194/acp-9-1579-2009. - DOI
-
- Amodio M., Catino S., Dambruoso P.R., De Gennaro G., Di Gilio A., Giungato P., Laiola E., Marzocca A., Mazzone A., Sardaro A., et al. Atmospheric deposition: Sampling procedures, analytical methods, and main recent findings from the scientific literature. Adv. Meteorol. 2014;2014:161730. doi: 10.1155/2014/161730. - DOI
-
- Schlesinger W.H. Biogeochemistry: An Analysis of Global Change. Academic Press; New York, NY, USA: 1997.
-
- Tang A., Zhuang G., Wang Y., Yuan H., Sun Y. The chemistry of precipitation and its relation to aerosol in Beijing. Atmos. Environ. 2005;39:3397–3406. doi: 10.1016/j.atmosenv.2005.02.001. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

