Short- and Long-Term Stability of Aromatic Amines in Human Urine
- PMID: 36901145
- PMCID: PMC10002391
- DOI: 10.3390/ijerph20054135
Short- and Long-Term Stability of Aromatic Amines in Human Urine
Abstract
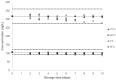
Several aromatic amines (AAs) are established by the International Agency for Research on Cancer as carcinogenic (group 1) or probable/possible carcinogens to humans (group 2A/2B). AAs can be found in mainstream and sidestream smoke from combustible tobacco products, as well as in certain environmental pollution and occupational exposure from several chemical industry sectors. Exposure to AAs can be estimated by measuring their concentrations in urine; however, information about the short-term and long-term stabilities of AAs in urine need to be characterized before conducting large-scale population studies on AA exposure and the potentially harmful effects of AA exposure. In this report, the storage stability of o-toluidine, 2,6-dimethylaniline, o-anisidine, 1-aminonaphthalene, 2-aminonaphthalene, and 4-aminobiphenyl fortified in pooled, filtered, non-smokers' urine is analyzed by isotope dilution gas chromatography-triple quadrupole mass spectrometry (ID GC-MS/MS). The six AAs were measured in urine samples stored at ~20 °C (collection temperature), 4 °C and 10 °C (short-term transit temperatures), and -20 °C and -70 °C (long-term storage temperatures) over a 10-day period. All six analytes were stable for 10 days at transit and long-term storage temperatures but showed reduced recovery at 20 °C. The instability of the target AAs at 20 °C suggests that immediate storage of freshly voided urine at low temperatures is needed to attenuate degradation. A subset of the urine samples was analyzed following a longer storage duration at -70 °C: all AAs were stable for up to 14 months at this temperature. The stability of the six AAs in urine samples can be maintained at the various temperature levels and storage times expected in a typical study set.
Keywords: aromatic amines; human urine; long-term stability; short-term stability.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
A New Automated Method for the Analysis of Aromatic Amines in Human Urine by GC-MS/MS.J Anal Toxicol. 2019 Jan 1;43(1):25-35. doi: 10.1093/jat/bky045. J Anal Toxicol. 2019. PMID: 30010885 Free PMC article.
-
Determination of three carcinogenic aromatic amines in urine of smokers and nonsmokers.J Anal Toxicol. 2006 Apr;30(3):187-95. doi: 10.1093/jat/30.3.187. J Anal Toxicol. 2006. PMID: 16803653
-
Detection of carcinogenic aromatic amines in the urine of non-smokers.Sci Total Environ. 2000 Feb 28;247(1):81-90. doi: 10.1016/s0048-9697(99)00471-4. Sci Total Environ. 2000. PMID: 10721145
-
[Aromatic amines with certain, probable, or possible carcinogenic action: reference values].G Ital Med Lav Ergon. 2003 Jan-Mar;25(1):68-73. G Ital Med Lav Ergon. 2003. PMID: 12696487 Review. Italian.
-
Biomonitoring of exposure to aromatic amines: haemoglobin adducts in humans.J Chromatogr B Analyt Technol Biomed Life Sci. 2002 Oct 5;778(1-2):49-62. doi: 10.1016/s0378-4347(01)00466-2. J Chromatogr B Analyt Technol Biomed Life Sci. 2002. PMID: 12376116 Review.
Cited by
-
Comparison of gas chromatographic techniques for the analysis of iodinated derivatives of aromatic amines.Anal Bioanal Chem. 2023 Jul;415(17):3313-3325. doi: 10.1007/s00216-023-04713-8. Epub 2023 May 20. Anal Bioanal Chem. 2023. PMID: 37208487 Free PMC article.
References
-
- Tang D., Warburton D., Tannenbaum S.R., Skipper P., Santella R.M., Cereijido G.S., Crawford F.G., Perera F.P. Molecular and genetic damage from environmental tobacco smoke in young children. Cancer Epidemiol. Biomark. Prev. 1999;8:427–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

