Ellagic Acid Prevents Particulate Matter-Induced Pulmonary Inflammation and Hyperactivity in Mice: A Pilot Study
- PMID: 36901532
- PMCID: PMC10001477
- DOI: 10.3390/ijerph20054523
Ellagic Acid Prevents Particulate Matter-Induced Pulmonary Inflammation and Hyperactivity in Mice: A Pilot Study
Abstract
The inhalation of fine particulate matter (PM) is a significant health-related environmental issue. Previously, we demonstrated that repeated PM exposure causes hyperlocomotive activity in mice, as well as inflammatory and hypoxic responses in their lungs. In this study, we evaluated the potential efficacy of ellagic acid (EA), a natural polyphenolic compound, against PM-induced pulmonary and behavioral abnormalities in mice. Four treatment groups were assigned in this study (n = 8): control (CON), particulate-matter-instilled (PMI), low-dose EA with PMI (EL + PMI), and high-dose EA with PMI (EH + PMI). EA (20 and 100 mg/kg body weight for low dose and high dose, respectively) was orally administered for 14 days in C57BL/6 mice, and after the eighth day, PM (5 mg/kg) was intratracheally instilled for 7 consecutive days. PM exposure induced inflammatory cell infiltration in the lungs following EA pretreatment. Moreover, PM exposure induced inflammatory protein expression in the bronchoalveolar lavage fluid and the expression of inflammatory (tumor necrosis factor alpha (Tnfα), interleukin (Il)-1b, and Il-6) and hypoxic (vascular endothelial growth factor alpha (Vegfα), ankyrin repeat domain 37 (Ankrd37)) response genes. However, EA pretreatment markedly prevented the induction of expression of inflammatory and hypoxic response genes in the lungs. Furthermore, PM exposure significantly triggered hyperactivity by increasing the total moving distance with an increase in moving speed in the open field test. On the contrary, EA pretreatment significantly prevented PM-induced hyperactivity. In conclusion, dietary intervention with EA may be a potential strategy to prevent PM-induced pathology and activity.
Keywords: ellagic acid; hyperactivity; hypoxia; inflammation; particulate matter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



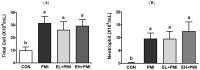
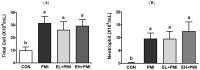




References
-
- Morsi A.A., Fouad H., Alasmari W.A., Faruk E.M. The biomechanistic aspects of renal cortical injury induced by diesel exhaust particles in rats and the renoprotective contribution of quercetin pretreatment: Histological and biochemical study. Environ. Toxicol. 2022;37:310–321. doi: 10.1002/tox.23399. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

