Prader-Willi Syndrome and Chromosome 15q11.2 BP1-BP2 Region: A Review
- PMID: 36901699
- PMCID: PMC10002205
- DOI: 10.3390/ijms24054271
Prader-Willi Syndrome and Chromosome 15q11.2 BP1-BP2 Region: A Review
Abstract
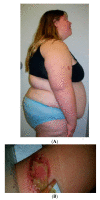
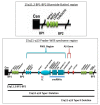
Prader-Willi syndrome (PWS) is a complex genetic disorder with three PWS molecular genetic classes and presents as severe hypotonia, failure to thrive, hypogonadism/hypogenitalism and developmental delay during infancy. Hyperphagia, obesity, learning and behavioral problems, short stature with growth and other hormone deficiencies are identified during childhood. Those with the larger 15q11-q13 Type I deletion with the absence of four non-imprinted genes (NIPA1, NIPA2, CYFIP1, TUBGCP5) from the 15q11.2 BP1-BP2 region are more severely affected compared with those with PWS having a smaller Type II deletion. NIPA1 and NIPA2 genes encode magnesium and cation transporters, supporting brain and muscle development and function, glucose and insulin metabolism and neurobehavioral outcomes. Lower magnesium levels are reported in those with Type I deletions. The CYFIP1 gene encodes a protein associated with fragile X syndrome. The TUBGCP5 gene is associated with attention-deficit hyperactivity disorder (ADHD) and compulsions, more commonly seen in PWS with the Type I deletion. When the 15q11.2 BP1-BP2 region alone is deleted, neurodevelopment, motor, learning and behavioral problems including seizures, ADHD, obsessive-compulsive disorder (OCD) and autism may occur with other clinical findings recognized as Burnside-Butler syndrome. The genes in the 15q11.2 BP1-BP2 region may contribute to more clinical involvement and comorbidities in those with PWS and Type I deletions.
Keywords: 15q11.2 BP1-BP2 deletion; PWS molecular genetic classes; Prader–Willi syndrome (PWS); Type II deletions; clinical findings; typical 15q11-q13 Type I.
Conflict of interest statement
There are no conflict of interest from the author.
Figures

References
-
- Butler M.G., Hartin S.N., Hossain W.A., Manzardo A.M., Kimonis V., Dykens E., Gold J.A., Kim S.J., Weisensel N., Tamura R., et al. Molecular genetic classification in Prader-Willi syndrome: A multisite cohort study. J. Med. Genet. 2019;56:149–153. doi: 10.1136/jmedgenet-2018-105301. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

