TERT Extra-Telomeric Roles: Antioxidant Activity and Mitochondrial Protection
- PMID: 36901881
- PMCID: PMC10002448
- DOI: 10.3390/ijms24054450
TERT Extra-Telomeric Roles: Antioxidant Activity and Mitochondrial Protection
Abstract
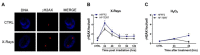
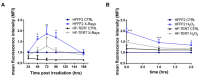
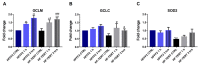
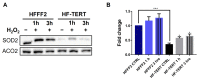
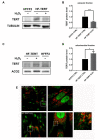
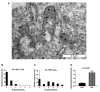
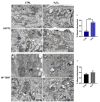
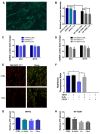
Telomerase reverse transcriptase (TERT) is the catalytic subunit of telomerase holoenzyme, which adds telomeric DNA repeats on chromosome ends to counteract telomere shortening. In addition, there is evidence of TERT non-canonical functions, among which is an antioxidant role. In order to better investigate this role, we tested the response to X-rays and H2O2 treatment in hTERT-overexpressing human fibroblasts (HF-TERT). We observed in HF-TERT a reduced induction of reactive oxygen species and an increased expression of the proteins involved in the antioxidant defense. Therefore, we also tested a possible role of TERT inside mitochondria. We confirmed TERT mitochondrial localization, which increases after oxidative stress (OS) induced by H2O2 treatment. We next evaluated some mitochondrial markers. The basal mitochondria quantity appeared reduced in HF-TERT compared to normal fibroblasts and an additional reduction was observed after OS; nevertheless, the mitochondrial membrane potential and morphology were better conserved in HF-TERT. Our results suggest a protective function of TERT against OS, also preserving mitochondrial functionality.
Keywords: electron microscopy; mitochondrion; oxidative response; primary cell lines; telomerase catalytic subunit.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Olovnikov A.M. Principle of marginotomy in template synthesis of polynucleotides. Dokl. Akad. Nauk SSSR. 1971;201:1496–1499. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

