Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials
- PMID: 36901908
- PMCID: PMC10003109
- DOI: 10.3390/ijms24054476
Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials
Abstract
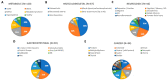
Medicinal properties of turmeric (Curcuma longa L.), a plant used for centuries as an anti-inflammatory, are attributed to its polyphenolic curcuminoids, where curcumin predominates. Although "curcumin" supplements are a top-selling botanical with promising pre-clinical effects, questions remain regarding biological activity in humans. To address this, a scoping review was conducted to assess human clinical trials reporting oral curcumin effects on disease outcomes. Eight databases were searched using established guidelines, yielding 389 citations (from 9528 initial) that met inclusion criteria. Half focused on obesity-associated metabolic disorders (29%) or musculoskeletal disorders (17%), where inflammation is a key driver, and beneficial effects on clinical outcomes and/or biomarkers were reported for most citations (75%) in studies that were primarily double-blind, randomized, and placebo-controlled trials (77%, D-RCT). Citations for the next most studied disease categories (neurocognitive [11%] or gastrointestinal disorders [10%], or cancer [9%]), were far fewer in number and yielded mixed results depending on study quality and condition studied. Although additional research is needed, including systematic evaluation of diverse curcumin formulations and doses in larger D-RCT studies, the preponderance of current evidence for several highly studied diseases (e.g., metabolic syndrome, osteoarthritis), which are also clinically common, are suggestive of clinical benefits.
Keywords: Curcuma longa L.; curcumin; curcuminoids; dietary supplement; human clinical trials; turmeric.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
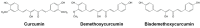



References
-
- Velayudhan K.C., Dikshit N., Nizar M.A. Ethnobotany of turmeric (Curcuma longa L.) Indian J. Tradit. Knowl. 2012;11:607–614.
-
- Pawar H.A., Karde M., Mundle N., Jadhav P.R., Mehra K. Phytochemical Evaluation and Curcumin Content Determination of Turmeric Rhizomes Collected From Bhandara District of Maharashtra (India) Med. Chem. 2014;4:588–591. doi: 10.4172/2161-0444.1000198. - DOI
-
- Skiba M.B., Luis P.B., Alfafara C., Billheimer D., Schneider C., Funk J.L. Curcuminoid Content and Safety-Related Markers of Quality of Turmeric Dietary Supplements Sold in an Urban Retail Marketplace in the United States. Mol. Nutr. Food Res. 2018;62:1800143. doi: 10.1002/mnfr.201800143. - DOI - PMC - PubMed
-
- Bates J. Oilseeds, spices, fruits and flavour in the Indus Civilisation. J. Archaeol. Sci. Rep. 2019;24:879–887. doi: 10.1016/j.jasrep.2019.02.033. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

