miR-210 Expression Is Strongly Hypoxia-Induced in Anaplastic Thyroid Cancer Cell Lines and Is Associated with Extracellular Vesicles and Argonaute-2
- PMID: 36901936
- PMCID: PMC10002857
- DOI: 10.3390/ijms24054507
miR-210 Expression Is Strongly Hypoxia-Induced in Anaplastic Thyroid Cancer Cell Lines and Is Associated with Extracellular Vesicles and Argonaute-2
Abstract
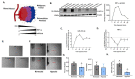
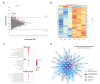
Hypoxia, or low oxygen tension, is frequently found in highly proliferative solid tumors such as anaplastic thyroid carcinoma (ATC) and is believed to promote resistance to chemotherapy and radiation. Identifying hypoxic cells for targeted therapy may thus be an effective approach to treating aggressive cancers. Here, we explore the potential of the well-known hypoxia-responsive microRNA (miRNA) miR-210-3p as a cellular and extracellular biological marker of hypoxia. We compare miRNA expression across several ATC and papillary thyroid cancer (PTC) cell lines. In the ATC cell line SW1736, miR-210-3p expression levels indicate hypoxia during exposure to low oxygen conditions (2% O2). Furthermore, when released by SW1736 cells into the extracellular space, miR-210-3p is associated with RNA carriers such as extracellular vesicles (EVs) and Argonaute-2 (AGO2), making it a potential extracellular marker for hypoxia.
Keywords: HIF1-alpha; anaplastic thyroid cancer; extracellular vesicles; hypoxia; miR-210; miRNA; microRNA.
Conflict of interest statement
The authors declare no conflict of interest. Andrey Turchinovich is the co-founder and a shareholder of commercial company, Heidelberg Biolabs GmbH which provides data analysis services for Johns Hopkins University School of Medicine.
Figures


References
-
- Smallridge R.C., Ain K.B., Asa S.L., Bible K.C., Brierley J.D., Burman K.D., Kebebew E., Lee N.Y., Nikiforov Y.E., Rosenthal M.S., et al. American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid. 2012;22:1104–1139. doi: 10.1089/thy.2012.0302. - DOI - PubMed
-
- Hundahl S.A., Cady B., Cunningham M.P., Mazzaferri E., McKee R.F., Rosai J., Shah J.P., Fremgen A.M., Stewart A.K., Hölzer S. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the united states during 1996. Cancer. 2000;89:202–217. doi: 10.1002/1097-0142(20000701)89:1<202::AID-CNCR27>3.0.CO;2-A. - DOI - PubMed
-
- Burrows N., Williams J., Telfer B.A., Resch J., Valentine H.R., Fitzmaurice R.J., Eustace A., Irlam J., Rowling E.J., Hoang-Vu C., et al. Phosphatidylinositide 3-kinase (PI3K) and PI3K-related kinase (PIKK) activity contributes to radioresistance in thyroid carcinomas. Oncotarget. 2016;7:63106–63123. doi: 10.18632/oncotarget.11056. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

