Multiphoton FLIM Analyses of Native and UVA-Modified Synthetic Melanins
- PMID: 36901948
- PMCID: PMC10002570
- DOI: 10.3390/ijms24054517
Multiphoton FLIM Analyses of Native and UVA-Modified Synthetic Melanins
Abstract
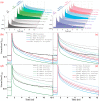
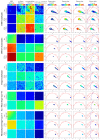
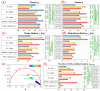
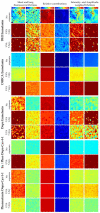
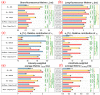
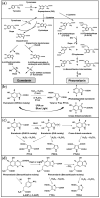
To better understand the impact of solar light exposure on human skin, the chemical characterization of native melanins and their structural photo-modifications is of central interest. As the methods used today are invasive, we investigated the possibility of using multiphoton fluorescence lifetime (FLIM) imaging, along with phasor and bi-exponential fitting analyses, as a non-invasive alternative method for the chemical analysis of native and UVA-exposed melanins. We demonstrated that multiphoton FLIM allows the discrimination between native DHI, DHICA, Dopa eumelanins, pheomelanin, and mixed eu-/pheo-melanin polymers. We exposed melanin samples to high UVA doses to maximize their structural modifications. The UVA-induced oxidative, photo-degradation, and crosslinking changes were evidenced via an increase in fluorescence lifetimes along with a decrease in their relative contributions. Moreover, we introduced a new phasor parameter of a relative fraction of a UVA-modified species and provided evidence for its sensitivity in assessing the UVA effects. Globally, the fluorescence lifetime properties were modulated in a melanin-dependent and UVA dose-dependent manner, with the strongest modifications being observed for DHICA eumelanin and the weakest for pheomelanin. Multiphoton FLIM phasor and bi-exponential analyses hold promising perspectives for in vivo human skin mixed melanins characterization under UVA or other sunlight exposure conditions.
Keywords: BZ-AA pure benzothiazine; DHBTCA pure benzothiazole; DHI; DHICA; Dopa; Dopa-Cys; HPLC chemical analysis; UVA exposure; eumelanin; mixed eu-/pheo-melanins; multiphoton FLIM imaging; phasor and bi-exponential fitting analyses; pheomelanin.
Conflict of interest statement
The authors declare that they have no competing financial interest. A.-M.P., T.B., S.B. and S.D.B. are employees of L’Oréal Research and Innovation. S.I. is consulting for L’Oréal Research and Innovation.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

