Drugging the PI3K/AKT/mTOR Pathway in ER+ Breast Cancer
- PMID: 36901954
- PMCID: PMC10003259
- DOI: 10.3390/ijms24054522
Drugging the PI3K/AKT/mTOR Pathway in ER+ Breast Cancer
Abstract
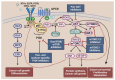
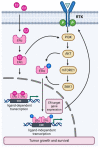
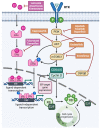
The frequent activation of the PI3K/AKT/mTOR pathway and its crucial role in estrogen receptor-positive (ER+) breast cancer tumorigenesis and drug resistance has made it a highly attractive therapeutic target in this breast cancer subtype. Consequently, the number of new inhibitors in clinical development targeting this pathway has drastically increased. Among these, the PIK3CA isoform-specific inhibitor alpelisib and the pan-AKT inhibitor capivasertib were recently approved in combination with the estrogen receptor degrader fulvestrant for the treatment of ER+ advanced breast cancer after progression on an aromatase inhibitor. Nevertheless, the clinical development of multiple inhibitors of the PI3K/AKT/mTOR pathway, in parallel with the incorporation of CDK4/6 inhibitors into the standard of care treatment in ER+ advanced breast cancer, has led to a multitude of available therapeutic agents and many possible combined strategies which complicate personalizing treatment. Here, we review the role of the PI3K/AKT/mTOR pathway in ER+ advanced breast cancer, highlighting the genomic contexts in which the various inhibitors of this pathway may have superior activity. We also discuss selected trials with agents targeting the PI3K/AKT/mTOR and related pathways as well as the rationale supporting the clinical development of triple combination therapy targeting ER, CDK4/6 and PI3K/AKT/mTOR in ER+ advanced breast cancer.
Keywords: CDK4/6 inhibitor; PI3K/AKT/mTOR pathway; estrogen receptor-positive breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Di Nicolantonio F., Arena S., Tabernero J., Grosso S., Molinari F., Macarulla T., Russo M., Cancelliere C., Zecchin D., Mazzucchelli L. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J. Clin. Investig. 2010;120:2858–2866. doi: 10.1172/JCI37539. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

