Evidence for Extensive Duplication and Subfunctionalization of FCRL6 in Armadillo (Dasypus novemcinctus)
- PMID: 36901962
- PMCID: PMC10003336
- DOI: 10.3390/ijms24054531
Evidence for Extensive Duplication and Subfunctionalization of FCRL6 in Armadillo (Dasypus novemcinctus)
Abstract
The control of infections by the vertebrate adaptive immune system requires careful modulation to optimize defense and minimize harm to the host. The Fc receptor-like (FCRL) genes encode immunoregulatory molecules homologous to the receptors for the Fc portion of immunoglobulin (FCR). To date, nine different genes (FCRL1-6, FCRLA, FCRLB and FCRLS) have been identified in mammalian organisms. FCRL6 is located at a separate chromosomal position from the FCRL1-5 locus, has conserved synteny in mammals and is situated between the SLAMF8 and DUSP23 genes. Here, we show that this three gene block underwent repeated duplication in Dasypus novemcinctus (nine-banded armadillo) resulting in six FCRL6 copies, of which five appear functional. Among 21 mammalian genomes analyzed, this expansion was unique to D. novemcinctus. Ig-like domains that derive from the five clustered FCRL6 functional gene copies show high structural conservation and sequence identity. However, the presence of multiple non-synonymous amino acid changes that would diversify individual receptor function has led to the hypothesis that FCRL6 endured subfunctionalization during evolution in D. novemcinctus. Interestingly, D. novemcinctus is noteworthy for its natural resistance to the Mycobacterium leprae pathogen that causes leprosy. Because FCRL6 is chiefly expressed by cytotoxic T and NK cells, which are important in cellular defense responses against M. leprae, we speculate that FCRL6 subfunctionalization could be relevant for the adaptation of D. novemcinctus to leprosy. These findings highlight the species-specific diversification of FCRL family members and the genetic complexity underlying evolving multigene families critical for modulating adaptive immune protection.
Keywords: FCRL; FCRL6; armadillo; gene duplication; leprosy; subfunctionalization.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

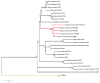
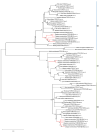

References
-
- Ehrhardt G.R.A., Cooper M.D. Immunoregulatory Roles for Fc Receptor-Like Molecules. In: Ahmed R., Honjo T., editors. Negative Co-Receptors and Ligands. Springer; Berlin/Heidelberg, Germany: 2011. pp. 89–104. - PubMed
MeSH terms
Substances
Grants and funding
- NORTE-01-0246-FEDER-000063/Norte Portugal Regional Operational Programme (NORTE2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF)
- SFRH/BPD/117451/2016/Fundação para a Ciência e Tecnologia
- CEECIND/CP1601/CT0005/Fundação para a Ciência e Tecnologia
LinkOut - more resources
Full Text Sources
Medical
Research Materials

