Cytotoxic and Bactericidal Effects of Inhalable Ciprofloxacin-Loaded Poly(2-ethyl-2-oxazoline) Nanoparticles with Traces of Zinc Oxide
- PMID: 36901963
- PMCID: PMC10002581
- DOI: 10.3390/ijms24054532
Cytotoxic and Bactericidal Effects of Inhalable Ciprofloxacin-Loaded Poly(2-ethyl-2-oxazoline) Nanoparticles with Traces of Zinc Oxide
Abstract
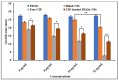
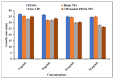
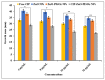
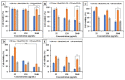
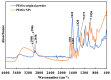
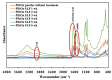
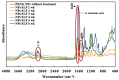
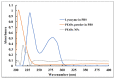
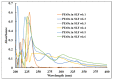
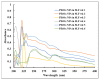
The bactericidal effects of inhalable ciprofloxacin (CIP) loaded-poly(2-ethyl-2-oxazoline) (PEtOx) nanoparticles (NPs) with traces of zinc oxide (ZnO) were investigated against clinical strains of the respiratory pathogens Staphylococcus aureus and Pseudomonas aeruginosa. CIP-loaded PEtOx NPs retained their bactericidal activity within the formulations compared to free CIP drugs against these two pathogens, and bactericidal effects were enhanced with the inclusion of ZnO. PEtOx polymer and ZnO NPs did not show bactericidal activity alone or in combination against these pathogens. The formulations were tested to determine the cytotoxic and proinflammatory effects on airway epithelial cells derived from healthy donors (NHBE), donors with chronic obstructive pulmonary disease (COPD, DHBE), and a cell line derived from adults with cystic fibrosis (CFBE41o-) and macrophages from healthy adult controls (HCs), and those with either COPD or CF. NHBE cells demonstrated maximum cell viability (66%) against CIP-loaded PEtOx NPs with the half maximal inhibitory concentration (IC50) value of 50.7 mg/mL. CIP-loaded PEtOx NPs were more toxic to epithelial cells from donors with respiratory diseases than NHBEs, with respective IC50 values of 0.103 mg/mL for DHBEs and 0.514 mg/mL for CFBE41o- cells. However, high concentrations of CIP-loaded PEtOx NPs were toxic to macrophages, with respective IC50 values of 0.002 mg/mL for HC macrophages and 0.021 mg/mL for CF-like macrophages. PEtOx NPs, ZnO NPs, and ZnO-PEtOx NPs with no drug were not cytotoxic to any cells investigated. The in vitro digestibility of PEtOx and its NPs was investigated in simulated lung fluid (SLF) (pH 7.4). The analysed samples were characterized using Fourier transform infrared spectroscopy (ATR-FTIR), scanning electron microscopy (SEM), and UV-Vis spectroscopy. Digestion of PEtOx NPs commenced one week following incubation and was completely digested after four weeks; however, the original PEtOx was not digested after six weeks of incubation. The outcome of this study revealed that PEtOx polymer could be considered an efficient drug delivery carrier in respiratory linings, and CIP-loaded PEtOx NPs with traces of ZnO could be a promising addition to inhalable treatments against resistant bacteria with reduced toxicity.
Keywords: antibiotic resistance; antimicrobial agents; bactericidal effects; cytotoxicity; digestibility; inhalable formulation; nanoparticles; poly(2-ethyl-2-oxazoline); zinc oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Meisner S. Current management of lower respiratory tract infections. Prescriber. 2006;17:48–58. doi: 10.1002/psb.354. - DOI
-
- Deborah C., John P., Allan P., Paul B., Peter A., Alen W. Ciprofloxacin. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic use. Drugs. 1988;35:373–447. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

