Small Extracellular Vesicles Derived from Induced Pluripotent Stem Cells in the Treatment of Myocardial Injury
- PMID: 36902008
- PMCID: PMC10003569
- DOI: 10.3390/ijms24054577
Small Extracellular Vesicles Derived from Induced Pluripotent Stem Cells in the Treatment of Myocardial Injury
Abstract
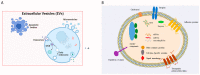
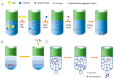
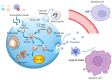
Induced pluripotent stem cell (iPSC) therapy brings great hope to the treatment of myocardial injuries, while extracellular vesicles may be one of the main mechanisms of its action. iPSC-derived small extracellular vesicles (iPSCs-sEVs) can carry genetic and proteinaceous substances and mediate the interaction between iPSCs and target cells. In recent years, more and more studies have focused on the therapeutic effect of iPSCs-sEVs in myocardial injury. IPSCs-sEVs may be a new cell-free-based treatment for myocardial injury, including myocardial infarction, myocardial ischemia-reperfusion injury, coronary heart disease, and heart failure. In the current research on myocardial injury, the extraction of sEVs from mesenchymal stem cells induced by iPSCs was widely used. Isolation methods of iPSCs-sEVs for the treatment of myocardial injury include ultracentrifugation, isodensity gradient centrifugation, and size exclusion chromatography. Tail vein injection and intraductal administration are the most widely used routes of iPSCs-sEV administration. The characteristics of sEVs derived from iPSCs which were induced from different species and organs, including fibroblasts and bone marrow, were further compared. In addition, the beneficial genes of iPSC can be regulated through CRISPR/Cas9 to change the composition of sEVs and improve the abundance and expression diversity of them. This review focused on the strategies and mechanisms of iPSCs-sEVs in the treatment of myocardial injury, which provides a reference for future research and the application of iPSCs-sEVs.
Keywords: exosome; extracellular vesicles; heart; induced pluripotent stem cells; mechanisms; myocardial injury.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
Similar articles
-
Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs.Circ Res. 2018 Jan 19;122(2):296-309. doi: 10.1161/CIRCRESAHA.117.311769. Epub 2017 Nov 8. Circ Res. 2018. PMID: 29118058 Free PMC article.
-
Inducible Pluripotent Stem Cell-Derived Small Extracellular Vesicles Rejuvenate Senescent Blood-Brain Barrier to Protect against Ischemic Stroke in Aged Mice.ACS Nano. 2023 Jan 10;17(1):775-789. doi: 10.1021/acsnano.2c10824. Epub 2022 Dec 23. ACS Nano. 2023. PMID: 36562422
-
Optimized culture methods for isolating small extracellular vesicles derived from human induced pluripotent stem cells.J Extracell Vesicles. 2021 Apr;10(6):e12065. doi: 10.1002/jev2.12065. Epub 2021 Apr 10. J Extracell Vesicles. 2021. PMID: 33868601 Free PMC article.
-
The Role of Small Extracellular Vesicles Derived from Mesenchymal Stromal Cells on Myocardial Protection: a Review of Current Advances and Future Perspectives.Cardiovasc Drugs Ther. 2024 Dec;38(6):1111-1122. doi: 10.1007/s10557-023-07472-x. Epub 2023 May 25. Cardiovasc Drugs Ther. 2024. PMID: 37227567 Free PMC article. Review.
-
Mesenchymal and Induced Pluripotent Stem Cells-Derived Extracellular Vesicles: The New Frontier for Regenerative Medicine?Cells. 2020 May 8;9(5):1163. doi: 10.3390/cells9051163. Cells. 2020. PMID: 32397132 Free PMC article. Review.
Cited by
-
Exosomes-mediated drug delivery for the treatment of myocardial injury.Ann Med Surg (Lond). 2023 Dec 2;86(1):292-299. doi: 10.1097/MS9.0000000000001473. eCollection 2024 Jan. Ann Med Surg (Lond). 2023. PMID: 38222684 Free PMC article. Review.
-
Stem Cell Therapy for Myocardial Infarction Recovery: Advances, Challenges, and Future Directions.Biomedicines. 2025 May 16;13(5):1209. doi: 10.3390/biomedicines13051209. Biomedicines. 2025. PMID: 40427036 Free PMC article. Review.
-
Emerging Trends and Innovations in the Treatment and Diagnosis of Atherosclerosis and Cardiovascular Disease: A Comprehensive Review towards Healthier Aging.Pharmaceutics. 2024 Aug 3;16(8):1037. doi: 10.3390/pharmaceutics16081037. Pharmaceutics. 2024. PMID: 39204382 Free PMC article. Review.
-
Stem Cell Therapy against Ischemic Heart Disease.Int J Mol Sci. 2024 Mar 28;25(7):3778. doi: 10.3390/ijms25073778. Int J Mol Sci. 2024. PMID: 38612587 Free PMC article. Review.
-
Extracellular vesicle therapeutics for cardiac repair.J Mol Cell Cardiol. 2025 Feb;199:12-32. doi: 10.1016/j.yjmcc.2024.11.005. Epub 2024 Nov 26. J Mol Cell Cardiol. 2025. PMID: 39603560 Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

