Organizational Principles of the Centrifugal Projections to the Olfactory Bulb
- PMID: 36902010
- PMCID: PMC10002860
- DOI: 10.3390/ijms24054579
Organizational Principles of the Centrifugal Projections to the Olfactory Bulb
Abstract
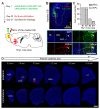
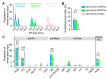
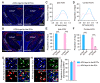
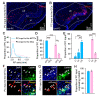
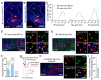
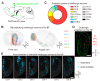
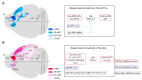
Centrifugal projections in the olfactory system are critical to both olfactory processing and behavior. The olfactory bulb (OB), the first relay station in odor processing, receives a substantial number of centrifugal inputs from the central brain regions. However, the anatomical organization of these centrifugal connections has not been fully elucidated, especially for the excitatory projection neurons of the OB, the mitral/tufted cells (M/TCs). Using rabies virus-mediated retrograde monosynaptic tracing in Thy1-Cre mice, we identified that the three most prominent inputs of the M/TCs came from the anterior olfactory nucleus (AON), the piriform cortex (PC), and the basal forebrain (BF), similar to the granule cells (GCs), the most abundant population of inhibitory interneurons in the OB. However, M/TCs received proportionally less input from the primary olfactory cortical areas, including the AON and PC, but more input from the BF and contralateral brain regions than GCs. Unlike organizationally distinct inputs from the primary olfactory cortical areas to these two types of OB neurons, inputs from the BF were organized similarly. Furthermore, individual BF cholinergic neurons innervated multiple layers of the OB, forming synapses on both M/TCs and GCs. Taken together, our results indicate that the centrifugal projections to different types of OB neurons may provide complementary and coordinated strategies in olfactory processing and behavior.
Keywords: centrifugal projections; granule cells; mitral/tufted cells; olfactory bulb; virus-mediated tracing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Diverse Representations of Olfactory Information in Centrifugal Feedback Projections.J Neurosci. 2016 Jul 13;36(28):7535-45. doi: 10.1523/JNEUROSCI.3358-15.2016. J Neurosci. 2016. PMID: 27413162 Free PMC article.
-
Functional imaging of cortical feedback projections to the olfactory bulb.Front Neural Circuits. 2014 Jul 3;8:73. doi: 10.3389/fncir.2014.00073. eCollection 2014. Front Neural Circuits. 2014. PMID: 25071454 Free PMC article.
-
CCKergic Tufted Cells Differentially Drive Two Anatomically Segregated Inhibitory Circuits in the Mouse Olfactory Bulb.J Neurosci. 2020 Aug 5;40(32):6189-6206. doi: 10.1523/JNEUROSCI.0769-20.2020. Epub 2020 Jun 30. J Neurosci. 2020. PMID: 32605937 Free PMC article.
-
Odor representation and coding by the mitral/tufted cells in the olfactory bulb.J Zhejiang Univ Sci B. 2024 Oct 15;25(10):824-840. doi: 10.1631/jzus.B2400051. J Zhejiang Univ Sci B. 2024. PMID: 39420520 Free PMC article. Review.
-
Centrifugal innervation of the mammalian olfactory bulb.Anat Sci Int. 2008 Dec;83(4):218-27. doi: 10.1111/j.1447-073X.2007.00223.x. Anat Sci Int. 2008. PMID: 19159349 Review.
Cited by
-
New Trends in Pathology: From Cell Morphology to Molecular Medicine.Int J Mol Sci. 2023 Jul 21;24(14):11743. doi: 10.3390/ijms241411743. Int J Mol Sci. 2023. PMID: 37511502 Free PMC article.
-
Altered whole-brain gray matter volume in form-deprivation myopia rats based on voxel-based morphometry: A pilot study.Front Neurosci. 2023 Apr 18;17:1113578. doi: 10.3389/fnins.2023.1113578. eCollection 2023. Front Neurosci. 2023. PMID: 37144093 Free PMC article.
-
[Application of optogenetic technology in the research on olfactory bulb neural projection from advanced brain regions to regulate olfactory signal processing].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2024 Dec 25;41(6):1265-1270. doi: 10.7507/1001-5515.202404009. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2024. PMID: 40000218 Free PMC article. Review. Chinese.
References
MeSH terms
Grants and funding
- 2018B030331001/Key-Area Research and Development Program of Guangdong Province
- XDB32030200/Strategic Priority Research Program of the Chinese Academy of Sciences
- 81661148053/National Natural Science Foundation of China
- 31830035/National Natural Science Foundation of China
- 31771156/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Miscellaneous

