S1P Released by SGPL1-Deficient Astrocytes Enhances Astrocytic ATP Production via S1PR2,4, Thus Keeping Autophagy in Check: Potential Consequences for Brain Health
- PMID: 36902011
- PMCID: PMC10003137
- DOI: 10.3390/ijms24054581
S1P Released by SGPL1-Deficient Astrocytes Enhances Astrocytic ATP Production via S1PR2,4, Thus Keeping Autophagy in Check: Potential Consequences for Brain Health
Abstract
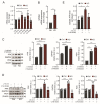
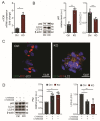
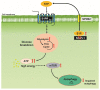
Astrocytes are critical players in brain health and disease. Sphingosine-1-phosphate (S1P), a bioactive signaling lipid, is involved in several vital processes, including cellular proliferation, survival, and migration. It was shown to be crucial for brain development. Its absence is embryonically lethal, affecting, inter alia, the anterior neural tube closure. However, an excess of S1P due to mutations in S1P-lyase (SGPL1), the enzyme responsible for its constitutive removal, is also harmful. Of note, the gene SGPL1 maps to a region prone to mutations in several human cancers and also in S1P-lyase insufficiency syndrome (SPLIS) characterized by several symptoms, including peripheral and central neurological defects. Here, we investigated the impact of S1P on astrocytes in a mouse model with the neural-targeted ablation of SGPL1. We found that SGPL1 deficiency, and hence the accumulation of its substrate, S1P, causes the elevated expression of glycolytic enzymes and preferentially directs pyruvate into the tricarboxylic acid (TCA) cycle through its receptors (S1PR2,4). In addition, the activity of TCA regulatory enzymes was increased, and consequently, so was the cellular ATP content. The high energy load activates the mammalian target of rapamycin (mTOR), thus keeping astrocytic autophagy in check. Possible consequences for the viability of neurons are discussed.
Keywords: S1P-lyase (SGPL1); SPLIS; autophagy; glucose metabolism; sphingosine 1-phosphate (S1P).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Neurodegeneration Caused by S1P-Lyase Deficiency Involves Calcium-Dependent Tau Pathology and Abnormal Histone Acetylation.Cells. 2020 Sep 28;9(10):2189. doi: 10.3390/cells9102189. Cells. 2020. PMID: 32998447 Free PMC article.
-
Sphingosine-1-phosphate-lyase deficiency affects glucose metabolism in a way that abets oncogenesis.Mol Oncol. 2022 Oct;16(20):3642-3653. doi: 10.1002/1878-0261.13300. Epub 2022 Aug 16. Mol Oncol. 2022. PMID: 35973936 Free PMC article.
-
Neural sphingosine 1-phosphate accumulation activates microglia and links impaired autophagy and inflammation.Glia. 2019 Oct;67(10):1859-1872. doi: 10.1002/glia.23663. Epub 2019 Jun 24. Glia. 2019. PMID: 31231866
-
Sphingosine phosphate lyase insufficiency syndrome (SPLIS): A novel inborn error of sphingolipid metabolism.Adv Biol Regul. 2019 Jan;71:128-140. doi: 10.1016/j.jbior.2018.09.004. Epub 2018 Sep 25. Adv Biol Regul. 2019. PMID: 30274713 Free PMC article. Review.
-
Genotype/Phenotype Interactions and First Steps Toward Targeted Therapy for Sphingosine Phosphate Lyase Insufficiency Syndrome.Cell Biochem Biophys. 2021 Sep;79(3):547-559. doi: 10.1007/s12013-021-01013-9. Epub 2021 Jun 16. Cell Biochem Biophys. 2021. PMID: 34133011 Review.
Cited by
-
Lysosomal acidification impairment in astrocyte-mediated neuroinflammation.J Neuroinflammation. 2025 Mar 10;22(1):72. doi: 10.1186/s12974-025-03410-w. J Neuroinflammation. 2025. PMID: 40065324 Free PMC article. Review.
-
Deciphering the role of sphingosine 1-phosphate in central nervous system myelination and repair.J Neurochem. 2025 Jan;169(1):e16228. doi: 10.1111/jnc.16228. Epub 2024 Sep 17. J Neurochem. 2025. PMID: 39290063 Free PMC article. Review.
-
S1P Lyase Deficiency in the Brain Promotes Astrogliosis and NLRP3 Inflammasome Activation via Purinergic Signaling.Cells. 2023 Jul 13;12(14):1844. doi: 10.3390/cells12141844. Cells. 2023. PMID: 37508508 Free PMC article.
-
The role of sphingosine-1-phosphate in autophagy and related disorders.Cell Death Discov. 2023 Oct 18;9(1):380. doi: 10.1038/s41420-023-01681-x. Cell Death Discov. 2023. PMID: 37852968 Free PMC article. Review.
References
-
- Futerman A.H. Biochemistry of Lipids and Membranes. 6th ed. Elsevier; Amsterdam, The Netherlands: 2016. Sphingolipids; pp. 297–326.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

