Micro-Evolutionary Processes in Armeria maritima at Metalliferous Sites
- PMID: 36902080
- PMCID: PMC10003435
- DOI: 10.3390/ijms24054650
Micro-Evolutionary Processes in Armeria maritima at Metalliferous Sites
Abstract
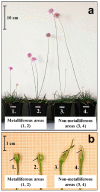
Tolerance to heavy metals in plants is a model process used to study adaptations to extremely unfavorable environments. One species capable of colonizing areas with high contents of heavy metals is Armeria maritima (Mill.) Wild. A. maritima plants growing in metalliferous areas differ in their morphological features and tolerance levels to heavy metals compared to individuals of the same species growing in non-metalliferous areas. The A. maritima adaptations to heavy metals occur at the organismal, tissue, and cellular levels (e.g., the retention of metals in roots, enrichment of the oldest leaves with metals, accumulation of metals in trichomes, and excretion of metals by salt glands of leaf epidermis). This species also undergoes physiological and biochemical adaptations (e.g., the accumulation of metals in vacuoles of the root's tannic cells and secretion of such compounds as glutathione, organic acids, or HSP17). This work reviews the current knowledge on A. maritima adaptations to heavy metals occurring in zinc-lead waste heaps and the species' genetic variation from exposure to such habitats. A. maritima is an excellent example of microevolution processes in plants inhabiting anthropogenically changed areas.
Keywords: Armeria maritima (Mill.) Willd; heavy metals; metalliferous areas; metallophyte; microevolution.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Armeria maritima from a calamine heap--initial studies on physiologic-metabolic adaptations to metal-enriched soil.Ecotoxicol Environ Saf. 2008 Feb;69(2):209-18. doi: 10.1016/j.ecoenv.2007.01.010. Epub 2007 Mar 27. Ecotoxicol Environ Saf. 2008. PMID: 17391761
-
Role of the salt glands of Armeria maritima (halophyte) in removal of lead from tissues.Environ Sci Pollut Res Int. 2024 May;31(25):37790-37809. doi: 10.1007/s11356-024-33624-z. Epub 2024 May 24. Environ Sci Pollut Res Int. 2024. PMID: 38787470 Free PMC article.
-
Divergent biology of facultative heavy metal plants.J Plant Physiol. 2017 Dec;219:45-61. doi: 10.1016/j.jplph.2017.08.014. Epub 2017 Sep 7. J Plant Physiol. 2017. PMID: 29028613 Review.
-
Comparison of In Vitro and In Planta Heavy Metal Tolerance and Accumulation Potential of Different Armeria maritima Accessions from a Dry Coastal Meadow.Plants (Basel). 2022 Aug 12;11(16):2104. doi: 10.3390/plants11162104. Plants (Basel). 2022. PMID: 36015407 Free PMC article.
-
Implications of metal accumulation mechanisms to phytoremediation.Environ Sci Pollut Res Int. 2009 Mar;16(2):162-75. doi: 10.1007/s11356-008-0079-z. Epub 2008 Dec 6. Environ Sci Pollut Res Int. 2009. PMID: 19067014 Review.
Cited by
-
MicroED: Unveiling the Structural Chemistry of Plant Biomineralisation.Molecules. 2024 Oct 17;29(20):4916. doi: 10.3390/molecules29204916. Molecules. 2024. PMID: 39459285 Free PMC article.
-
Island plants with newly discovered reproductive traits have higher capacity for uniparental reproduction, supporting Baker's law.Sci Rep. 2024 May 18;14(1):11392. doi: 10.1038/s41598-024-62065-4. Sci Rep. 2024. PMID: 38762587 Free PMC article.
-
Are Ophiolitic Substrates Drivers for Reticulate Evolution in Armeria (Plumbaginaceae)?Ecol Evol. 2025 Jun 12;15(6):e71525. doi: 10.1002/ece3.71525. eCollection 2025 Jun. Ecol Evol. 2025. PMID: 40519888 Free PMC article.
References
-
- Ashley M., Willson M., Pergams O., O’Dowd D., Gende S., Brown J. Evolutionarily enlightened management. Biol. Conserv. 2003;111:115–123. doi: 10.1016/S0006-3207(02)00279-3. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

