Toward a Shigella Vaccine: Opportunities and Challenges to Fight an Antimicrobial-Resistant Pathogen
- PMID: 36902092
- PMCID: PMC10003550
- DOI: 10.3390/ijms24054649
Toward a Shigella Vaccine: Opportunities and Challenges to Fight an Antimicrobial-Resistant Pathogen
Abstract
Shigellosis causes more than 200,000 deaths worldwide and most of this burden falls on Low- and Middle-Income Countries (LMICs), with a particular incidence in children under 5 years of age. In the last decades, Shigella has become even more worrisome because of the onset of antimicrobial-resistant strains (AMR). Indeed, the WHO has listed Shigella as one of the priority pathogens for the development of new interventions. To date, there are no broadly available vaccines against shigellosis, but several candidates are being evaluated in preclinical and clinical studies, bringing to light very important data and information. With the aim to facilitate the understanding of the state-of-the-art of Shigella vaccine development, here we report what is known about Shigella epidemiology and pathogenesis with a focus on virulence factors and potential antigens for vaccine development. We discuss immunity after natural infection and immunization. In addition, we highlight the main characteristics of the different technologies that have been applied for the development of a vaccine with broad protection against Shigella.
Keywords: AMR; O-antigens; Shigella; lipopolysaccharides; type III secretion system; vaccines.
Conflict of interest statement
This work was undertaken at the request of and sponsored by GlaxoSmithKline Biologicals SA. GSK Vaccines Institute for Global Health Srl is an affiliate of GlaxoSmithKline Biologicals SA. All authors are employees of the GSK group of companies.
Figures


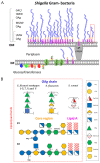
Similar articles
-
Shigellosis.J Microbiol. 2005 Apr;43(2):133-43. J Microbiol. 2005. PMID: 15880088 Review.
-
Challenges and opportunities in developing a Shigella-containing combination vaccine for children in low- and middle-income countries: Report of an expert convening.Vaccine. 2023 Apr 17;41(16):2634-2644. doi: 10.1016/j.vaccine.2023.03.003. Epub 2023 Mar 15. Vaccine. 2023. PMID: 36932030
-
Serum IgG antibodies to Shigella lipopolysaccharide antigens - a correlate of protection against shigellosis.Hum Vaccin Immunother. 2019;15(6):1401-1408. doi: 10.1080/21645515.2019.1606971. Epub 2019 May 9. Hum Vaccin Immunother. 2019. PMID: 31070988 Free PMC article. Review.
-
Recent progress towards development of a Shigella vaccine.Expert Rev Vaccines. 2013 Jan;12(1):43-55. doi: 10.1586/erv.12.135. Expert Rev Vaccines. 2013. PMID: 23256738 Review.
-
Vaccine value profile for Shigella.Vaccine. 2023 Nov 3;41 Suppl 2:S76-S94. doi: 10.1016/j.vaccine.2022.12.037. Epub 2023 Oct 10. Vaccine. 2023. PMID: 37827969
Cited by
-
Refining Immunogenicity through Intradermal Delivery of Outer Membrane Vesicles against Shigella flexneri in Mice.Int J Mol Sci. 2023 Nov 29;24(23):16910. doi: 10.3390/ijms242316910. Int J Mol Sci. 2023. PMID: 38069232 Free PMC article.
-
Phytochemical Analysis and Antimicrobial Activity of Terminalia bellirica (Gaertn.) Roxb. and Terminalia chebula Retz. Fruit Extracts Against Gastrointestinal Pathogens: Enhancing Antibiotic Efficacy.Microorganisms. 2024 Dec 22;12(12):2664. doi: 10.3390/microorganisms12122664. Microorganisms. 2024. PMID: 39770866 Free PMC article.
-
Protein-specific immune response elicited by the Shigella sonnei 1790GAHB GMMA-based candidate vaccine in adults with varying exposure to Shigella.mSphere. 2025 May 27;10(5):e0105724. doi: 10.1128/msphere.01057-24. Epub 2025 Apr 16. mSphere. 2025. PMID: 40237462 Free PMC article.
-
Comparison of Shigella GMMA and glycoconjugate four-component formulations in animals.Front Mol Biosci. 2023 Nov 16;10:1284515. doi: 10.3389/fmolb.2023.1284515. eCollection 2023. Front Mol Biosci. 2023. PMID: 38046812 Free PMC article.
-
Vaccines and Monoclonal Antibodies as Alternative Strategies to Antibiotics to Fight Antimicrobial Resistance.Int J Mol Sci. 2024 May 17;25(10):5487. doi: 10.3390/ijms25105487. Int J Mol Sci. 2024. PMID: 38791526 Free PMC article. Review.
References
-
- Dadonaite B., Ritchie H., Roser M. Diarrheal diseases. Our World Data. 2020
-
- Troeger C., Forouzanfar M., Rao P.C., Khalil I., Brown A., Reiner R.C., Fullman N., Thompson R., Abajobir A., Ahmed M., et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017;17:909–948. doi: 10.1016/S1473-3099(17)30276-1. - DOI - PMC - PubMed
-
- World Health Organization . Bacterial Vaccines in Clinical and Preclinical Development 2021: An Overview and Analysis. World Health Organization; Geneva, Switzerland: 2022.
-
- Shiga K. Observations on the epidemiology of dysentery in Japan. Philipp. J. Sci. 1906;1:485–500.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

