Manipulation of Oxidative Stress Responses by Non-Thermal Plasma to Treat Herpes Simplex Virus Type 1 Infection and Disease
- PMID: 36902102
- PMCID: PMC10003306
- DOI: 10.3390/ijms24054673
Manipulation of Oxidative Stress Responses by Non-Thermal Plasma to Treat Herpes Simplex Virus Type 1 Infection and Disease
Abstract
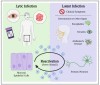
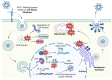
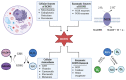
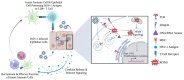
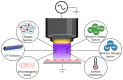
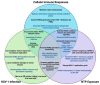
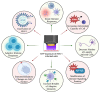
Herpes simplex virus type 1 (HSV-1) is a contagious pathogen with a large global footprint, due to its ability to cause lifelong infection in patients. Current antiviral therapies are effective in limiting viral replication in the epithelial cells to alleviate clinical symptoms, but ineffective in eliminating latent viral reservoirs in neurons. Much of HSV-1 pathogenesis is dependent on its ability to manipulate oxidative stress responses to craft a cellular environment that favors HSV-1 replication. However, to maintain redox homeostasis and to promote antiviral immune responses, the infected cell can upregulate reactive oxygen and nitrogen species (RONS) while having a tight control on antioxidant concentrations to prevent cellular damage. Non-thermal plasma (NTP), which we propose as a potential therapy alternative directed against HSV-1 infection, is a means to deliver RONS that affect redox homeostasis in the infected cell. This review emphasizes how NTP can be an effective therapy for HSV-1 infections through the direct antiviral activity of RONS and via immunomodulatory changes in the infected cells that will stimulate anti-HSV-1 adaptive immune responses. Overall, NTP application can control HSV-1 replication and address the challenges of latency by decreasing the size of the viral reservoir in the nervous system.
Keywords: adaptive immunity; antioxidant; antiviral therapy; immunomodulation; innate immunity; oxidative stress; plasma; reactive oxygen and nitrogen species; redox homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Non-Thermal Plasma Reduces HSV-1 Infection of and Replication in HaCaT Keratinocytes In Vitro.Int J Mol Sci. 2024 Mar 29;25(7):3839. doi: 10.3390/ijms25073839. Int J Mol Sci. 2024. PMID: 38612649 Free PMC article.
-
DLK-Dependent Biphasic Reactivation of Herpes Simplex Virus Latency Established in the Absence of Antivirals.J Virol. 2022 Jun 22;96(12):e0050822. doi: 10.1128/jvi.00508-22. Epub 2022 May 24. J Virol. 2022. PMID: 35608347 Free PMC article.
-
An M2 Rather than a TH2 Response Contributes to Better Protection against Latency Reactivation following Ocular Infection of Naive Mice with a Recombinant Herpes Simplex Virus 1 Expressing Murine Interleukin-4.J Virol. 2018 Apr 27;92(10):e00051-18. doi: 10.1128/JVI.00051-18. Print 2018 May 15. J Virol. 2018. PMID: 29491152 Free PMC article.
-
The Race between Host Antiviral Innate Immunity and the Immune Evasion Strategies of Herpes Simplex Virus 1.Microbiol Mol Biol Rev. 2020 Sep 30;84(4):e00099-20. doi: 10.1128/MMBR.00099-20. Print 2020 Nov 18. Microbiol Mol Biol Rev. 2020. PMID: 32998978 Free PMC article. Review.
-
Herpes Simplex Virus 1 Infection of Neuronal and Non-Neuronal Cells Elicits Specific Innate Immune Responses and Immune Evasion Mechanisms.Front Immunol. 2021 May 31;12:644664. doi: 10.3389/fimmu.2021.644664. eCollection 2021. Front Immunol. 2021. PMID: 34135889 Free PMC article. Review.
Cited by
-
Therapeutic Potential of Biochanin A in Herpes Simplex Keratitis.Pharmaceuticals (Basel). 2023 Sep 1;16(9):1240. doi: 10.3390/ph16091240. Pharmaceuticals (Basel). 2023. PMID: 37765049 Free PMC article.
-
Viral inactivation of murine coronavirus via multiple gas plasma-derived reactive species.Redox Biol. 2025 May;82:103591. doi: 10.1016/j.redox.2025.103591. Epub 2025 Mar 10. Redox Biol. 2025. PMID: 40085974 Free PMC article.
-
Investigation of β-Carboline Alkaloid Harmaline Against Cyvirus cyprinidallo3 Infection In Vitro and In Vivo.Viruses. 2025 May 9;17(5):687. doi: 10.3390/v17050687. Viruses. 2025. PMID: 40431698 Free PMC article.
-
Immunological Control of Herpes Simplex Virus Type 1 Infection: A Non-Thermal Plasma-Based Approach.Viruses. 2025 Apr 23;17(5):600. doi: 10.3390/v17050600. Viruses. 2025. PMID: 40431612 Free PMC article. Review.
-
Hyperoside inhibits EHV-8 infection via alleviating oxidative stress and IFN production through activating JNK/Keap1/Nrf2/HO-1 signaling pathways.J Virol. 2024 Apr 16;98(4):e0015924. doi: 10.1128/jvi.00159-24. Epub 2024 Mar 19. J Virol. 2024. PMID: 38499512 Free PMC article.
References
-
- Herpes Simplex Virus. [(accessed on 16 December 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

